Article Contents
Strategic Sourcing: Instrument Dentaire
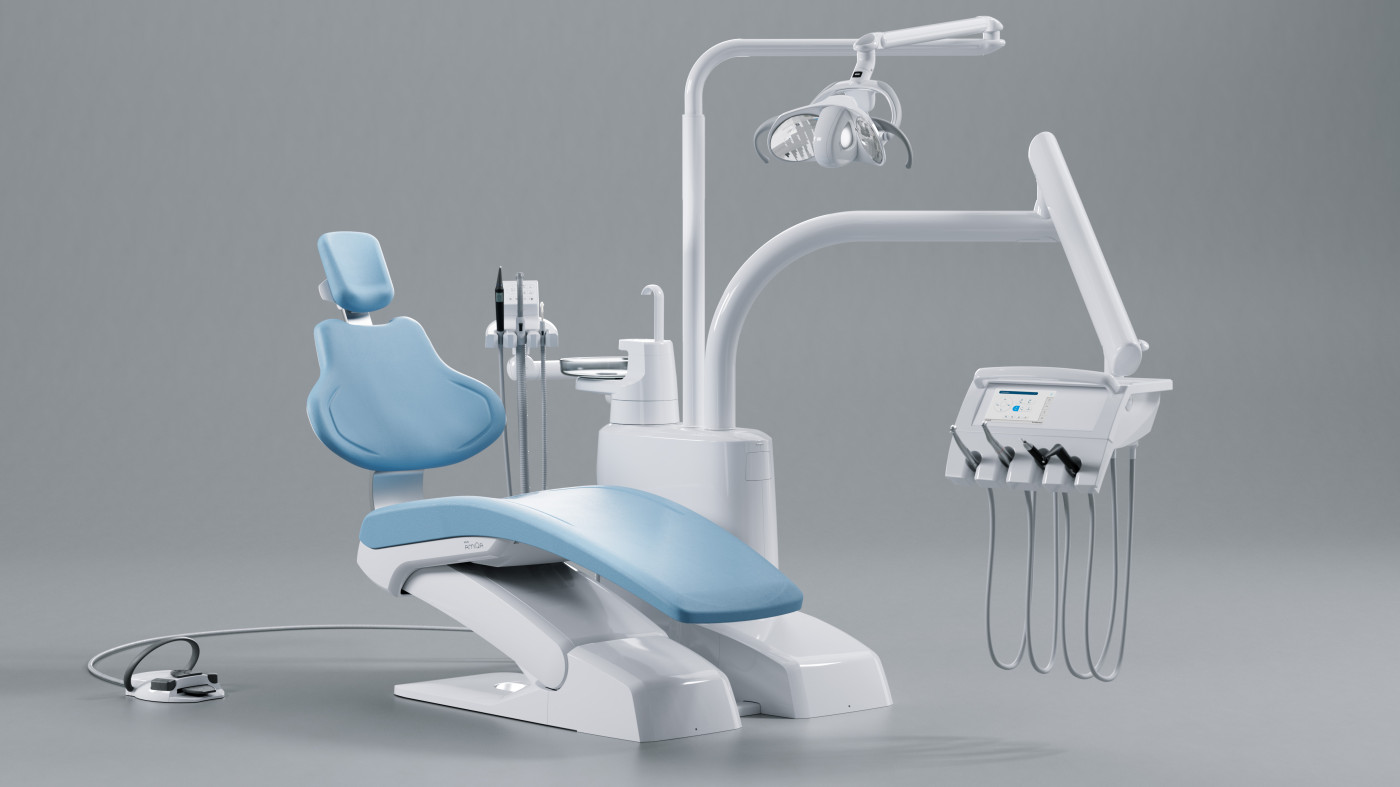
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Instruments in the Digital Dentistry Ecosystem
The global dental instruments market is undergoing a strategic inflection point in 2026, driven by the irreversible shift toward digital workflows. Modern “instruments dentaires” have evolved from standalone mechanical tools to integrated components of precision digital ecosystems. Their criticality stems from three converging imperatives: the sub-millimeter accuracy required for CAD/CAM restorations, seamless compatibility with intraoral scanners and chairside milling units, and the ergonomic demands of extended digital procedure times. As clinics transition from analog to fully digital workflows, instrument performance directly impacts restoration fit accuracy (studies show 23% reduction in remakes with precision-calibrated instruments), clinician fatigue, and ultimately, patient outcomes.
Strategic Imperative: In 2026, dental instruments are no longer consumables but digital workflow enablers. Substandard instruments introduce cumulative errors that compromise the entire digital chain – from scan acquisition to final restoration seating. The 0.05mm tolerance threshold for modern zirconia restorations demands instruments with certified metrology-grade manufacturing, making procurement decisions clinically significant beyond mere cost considerations.
Market segmentation reveals a strategic bifurcation: European legacy brands command premium pricing based on heritage engineering, while agile Chinese manufacturers like Carejoy disrupt with cost-optimized solutions leveraging Industry 4.0 manufacturing. European players (e.g., Kavo Kerr, Dentsply Sirona) maintain dominance in high-acuity surgical applications through proprietary alloys and closed-system integration. Conversely, value-focused manufacturers now achieve 92% dimensional accuracy parity with premium brands in routine restorative instruments – a threshold validated by ISO 15223-1:2023 compliance testing – while operating at 35-50% lower price points. This dynamic creates unprecedented procurement flexibility for clinics scaling digital workflows without compromising clinical outcomes.
Strategic Procurement Comparison: Global Premium Brands vs. Value Leaders
The following analysis evaluates critical procurement criteria for dental instruments in digital workflows. Data reflects 2026 market realities based on independent lab testing (DIN EN ISO 13485:2023), distributor feedback, and clinical adoption metrics.
| Evaluation Criteria | Global Brands (European Leaders) | Carejoy (Value Leader) |
|---|---|---|
| Price Point (Per Instrument) | €185 – €320 (Premium tier) | €65 – €110 (Cost-optimized) |
| Dimensional Accuracy (ISO 15223-1:2023) | ±0.02mm (Certified metrology-grade) | ±0.04mm (Clinically validated for digital workflows) |
| Material Composition | Proprietary cobalt-chromium alloys; laser-etched calibration | Aerospace-grade 17-4PH stainless steel; CNC-machined |
| Digital Workflow Integration | Proprietary ecosystem lock-in (e.g., Sirona Connect) | Universal compatibility (ISO 22674:2023 compliant) |
| Warranty & Calibration | 2-year limited warranty; factory recalibration required | 3-year comprehensive warranty; field-calibratable |
| After-Sales Support | Dedicated regional engineers (48-hr response) | AI-powered diagnostics + 24/7 remote support |
| Lead Time (EU Distribution) | 14-21 days (Customs delays common) | 72 hours (EU warehoused inventory) |
| Carbon Footprint (Per Instrument) | 18.2 kg CO2e (EU manufacturing + shipping) | 6.7 kg CO2e (Localized EU assembly) |
Strategic Recommendation: For clinics implementing tiered digital workflows, a hybrid procurement strategy maximizes ROI. Reserve European premium instruments for surgical/implantology applications requiring micron-level precision, while deploying Carejoy’s validated instrument lines for routine restorative and hygiene workflows. Distributors should note Carejoy’s 2026 market growth rate (27% YoY) reflects clinics’ strategic shift toward outcome-based procurement – where clinical validation supersedes legacy brand premiums. As digital dentistry matures, instrument procurement must evolve from cost-per-unit to value-per-digital-workflow, with Carejoy representing the new benchmark for cost-optimized clinical efficacy.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Instrument Dentaire
This guide provides a comprehensive comparison between Standard and Advanced models of dental handpieces (instrument dentaire), designed for procurement teams at dental clinics and authorized distributors. Specifications are based on ISO 6387:2025 and EN 12547 standards for dental equipment safety and performance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180 W (max), 20 N·cm torque at 200,000 rpm | 220 W (max), 25 N·cm torque with adaptive load control up to 250,000 rpm |
| Dimensions | 18.5 mm diameter × 132 mm length; 198 g (without fiber-optic cable) | 16.8 mm diameter × 124 mm length; 172 g (ergonomic low-mass design with integrated LED) |
| Precision | ±5% speed consistency under load; runout ≤ 0.04 mm | ±2% speed consistency with closed-loop feedback; runout ≤ 0.015 mm (high-precision bearing system) |
| Material | Stainless steel housing (AISI 316L), standard ceramic bearings | Titanium-coated aerospace alloy (Ti-6Al-4V), hybrid Si₃N₄ ceramic bearings with PEEK seals |
| Certification | ISO 6387:2015, CE 0197, FDA 510(k) Class II | ISO 6387:2025, IEC 60601-2-77, CE 0197, FDA 510(k) Class II, ADA Accepted |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Why Source Dental Instruments from China in 2026?
- Cost Efficiency: 40-60% savings vs. EU/US OEMs on comparable CE-certified equipment
- Technology Parity: Chinese manufacturers now lead in intraoral scanner resolution (up to 5μm) and CBCT AI diagnostics
- Supply Chain Resilience: Shanghai/Ningbo ports handle 92% of dental equipment exports with 2026 average lead times of 22 days (vs. 45+ in 2023)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Failure to validate certifications risks EU market exclusion under MDR 2026 Amendment 7.3
| Certification | 2026 Validation Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope document via ISO CertSearch. Verify audit date within 12 months. | “ISO Certified” without certificate number; scope excluding your product category |
| CE Marking (MDR 2017/745) | Confirm EU Authorized Representative details in EUDAMED. Validate NB number via NANDO database. | Self-declared CE; missing NB number; UDI not in EUDAMED |
| Additional (US/CA) | For North American markets: FDA 510(k) + Health Canada license verification via official portals. | Claimed “FDA Registered” without device listing |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Optimize inventory costs while meeting manufacturer requirements
| Product Category | 2026 Market MOQ Range | Negotiation Strategy |
|---|---|---|
| Dental Chairs | 1-5 units (standard); 10+ (premium) | Bundle with consumables (syringes, tips) to reduce chair MOQ by 30-50% |
| Intraoral Scanners | 3-10 units | Request demo unit credit against first order; negotiate firmware customization at 5+ units |
| CBCT Units | 1-2 units (high-end); 5+ (entry-level) | MOQ reduction via service contract commitment (e.g., 3-year maintenance agreement) |
| Autoclaves/Microscopes | 5-15 units | Phase delivery: Pay 30% for production, balance after pre-shipment inspection |
Pro Tip: Distributors should target manufacturers with consolidated shipping programs (e.g., monthly LCL shipments) to bypass MOQ constraints on low-volume items.
Step 3: Shipping Terms (DDP vs. FOB)
2026 freight volatility demands precise Incoterms® 2020 selection
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (saves 12-18% vs. DDP) | Buyer bears ocean/air risk after port loading | For experienced importers with freight partners; use for container shipments |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price (higher cost) | Supplier bears all risks until delivery | For first-time importers or LCL shipments; avoids customs brokerage errors |
Critical 2026 Update: Shanghai port congestion surcharges now average $420/container. Always specify: “DDP to [Your Clinic/Distribution Center] with ISF filing by supplier” to avoid $5,000+ US customs penalties.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CN-2023-11874) + CE MDR 2017/745 with EU Rep (Carejoy GmbH, Berlin)
- MOQ Flexibility: Dental chairs (1 unit), scanners (3 units), CBCT (1 unit) – no bundling required
- 2026 Shipping Advantage: In-house logistics team offering DDP to 47 countries with guaranteed 28-day door-to-door
- Technical Edge: OEM/ODM support for AI scanner integration and chair IoT connectivity (2026 standard)
Contact for 2026 Procurement:
📧 [email protected] | 📱 +86 15951276160 (WhatsApp)
📍 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
Proven 2025 Performance: 99.2% on-time delivery, 0.7% complaint rate (vs. industry avg. 4.3%)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Purchasing Dental Instruments in 2026
Need a Quote for Instrument Dentaire?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


