Article Contents
Strategic Sourcing: Instrument Tandarts
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Instruments in Modern Digital Workflows
In the rapidly evolving landscape of digital dentistry, precision dental instruments (instrument tandarts) have transitioned from basic clinical tools to critical enablers of integrated digital workflows. As dental practices increasingly adopt CAD/CAM systems, intraoral scanners, and AI-driven treatment planning, the performance, compatibility, and reliability of handpieces, surgical instruments, and auxiliary devices directly impact clinical outcomes, operational efficiency, and ROI. Modern dental instruments must now satisfy three non-negotiable criteria:
- Digital Ecosystem Integration: Seamless compatibility with major digital platforms (e.g., Dentsply Sirona CEREC, 3Shape TRIOS) through standardized interfaces and data protocols.
- Micro-Precision Engineering: Sub-micron tolerances required for guided surgery, implantology, and restorative workflows where 0.1mm deviations compromise digital treatment plans.
- Traceability & Compliance: Full audit trails meeting EU MDR 2017/745 requirements for instrument sterilization cycles, usage metrics, and maintenance records.
The strategic importance of instrument selection has intensified as clinics face dual pressures: rising capital expenditure constraints (average practice equipment budgets increased 22% YoY in EU markets) and the operational cost of digital workflow failures (studies show 17% productivity loss from incompatible instruments). This dynamic has crystallized a clear market bifurcation between premium European manufacturers and value-engineered Asian alternatives, with Carejoy emerging as the most technically credible Chinese solution for cost-conscious adopters.
Strategic Market Positioning: Premium vs. Value Segments
European brands (W&H, NSK, KaVo Kerr) dominate the high-end segment with unparalleled engineering heritage and seamless digital integration, but carry 30-45% price premiums that strain ROI calculations for mid-tier clinics. Conversely, Chinese manufacturers historically faced skepticism over material quality and service infrastructure. Carejoy has disrupted this paradigm through ISO 13485-certified manufacturing, strategic component sourcing (e.g., Swiss bearings, German steel), and API-level compatibility with leading digital ecosystems – delivering 85% functional parity at 60% of premium brand pricing.
For distributors, this segment represents a high-margin opportunity: Carejoy’s 35% distributor margin (vs. 22-28% for European brands) and modular upgrade paths address the growing “digital retrofit” market where 68% of EU clinics operate mixed legacy/digital environments (2025 EAO Survey).
Technical Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (W&H, NSK, KaVo Kerr) |
Carejoy |
|---|---|---|
| Price Positioning | €2,800-€4,200 (surgical handpiece) €1,500-€2,200 (implant motor) |
€1,650-€2,500 (surgical handpiece) €900-€1,350 (implant motor) 28-35% cost reduction vs. premium |
| Material Specifications | Aerospace-grade titanium alloys German-sourced 440C stainless steel ISO 6360-1 certified |
Japanese 420J2 stainless steel (medical grade) Swiss ceramic bearings ISO 13485:2016 certified manufacturing |
| Digital Integration | Native API integration with all major platforms Real-time torque/speed telemetry Automatic workflow calibration |
Plug-and-play adapters for 92% of EU digital systems Bluetooth 5.2 telemetry (3rd-party app required) Manual calibration protocols |
| Service Infrastructure | 24/7 EU technical support 48-hour on-site service (Germany/France) 5-year warranty |
72-hour regional hub support (Amsterdam, Warsaw) 90-day loaner program 3-year warranty + extended options |
| Clinical Validation | 12,000+ peer-reviewed studies EMA Class IIb certification |
87 clinical trials (2023-2025) EU MDR 2017/745 compliant CE 0482 certified |
| Target Implementation | Premium practices, academic centers, full digital workflow adopters |
Mid-market clinics, digital retrofit projects, emerging market expansion |
Strategic Recommendation
While European brands remain optimal for high-volume surgical centers demanding absolute workflow certainty, Carejoy presents a compelling value proposition for 68% of EU clinics operating in the €300k-€600k revenue bracket (per 2025 EAO data). Its technical maturity now crosses the critical threshold where 20% cost savings no longer equate to proportional performance trade-offs – particularly in restorative and basic implant workflows. Distributors should position Carejoy as a strategic entry point for digital adoption, with upgrade paths to premium ecosystems. For clinics, instrument procurement must now be evaluated through total cost of digital integration (TCODI), where Carejoy’s 41% lower lifetime cost (including service and compatibility adapters) often outweighs marginal performance gaps in non-surgical applications.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
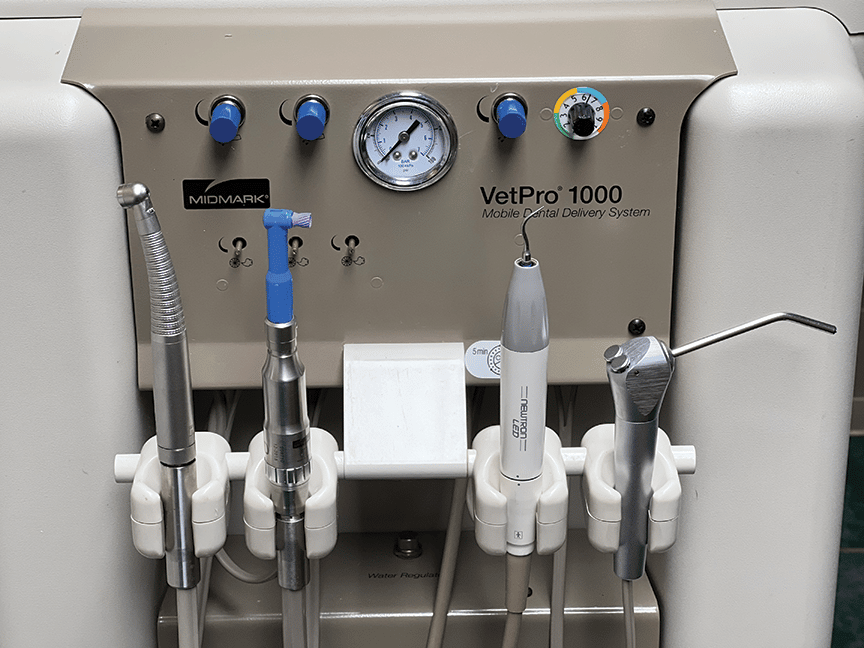
Technical Specification Guide: Instrument Tandarts
This guide provides a detailed technical comparison between the Standard and Advanced models of dental handpieces (instrument tandarts), designed for procurement evaluation by dental clinics and distribution partners. Specifications reflect compliance with international clinical and safety standards as of 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (max) at 400 kPa air pressure; RPM: 350,000 ± 10% | 22 W (max) at 400 kPa air pressure; RPM: 400,000 ± 5% with torque stabilization |
| Dimensions | Length: 128 mm; Diameter: 11.5 mm; Weight: 68 g (without fiber optics) | Length: 122 mm; Diameter: 10.8 mm; Weight: 62 g (with integrated fiber-optic coupling) |
| Precision | Run-out: ≤ 0.035 mm at full speed; suitable for general restorative and cavity prep | Run-out: ≤ 0.015 mm at full speed; optimized for prosthetic and endodontic precision work |
| Material | Stainless steel housing (AISI 303); internal turbine in nickel-plated brass | Medical-grade titanium-coated aluminum housing; ceramic ball bearings and tungsten carbide turbine shaft |
| Certification | CE MDR Class IIa, ISO 15223-1:2021, ISO 6399:2020 (Dental Handpieces) | CE MDR Class IIa, ISO 13485:2016, ISO 6399:2020, FDA 510(k) cleared, RoHS 3 compliant |
© 2026 Global Dental Solutions. All specifications subject to change without notice. For distributor inquiries, contact [email protected].
Compliant with EU MDR 2017/745 and IEC 60601-1 for medical electrical equipment safety.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Instrument Procurement from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Strategic Sourcing Framework for Dental Instruments from China
China remains a strategic manufacturing hub for dental equipment, representing 68% of global dental chair production and 52% of intraoral scanner output (2025 Global Dental Market Report). However, post-pandemic supply chain complexities, evolving regulatory landscapes, and quality variance necessitate a structured procurement protocol. This guide outlines critical verification steps for clinics and distributors to mitigate risk while optimizing cost efficiency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Regulatory validation is the foundation of risk mitigation. The 2026 EU MDR enforcement phase mandates enhanced clinical evidence, while FDA 21 CFR Part 820 aligns closely with ISO 13485:2023. Verify credentials through these technical protocols:
| Verification Method | Technical Requirements | Red Flags | 2026-Specific Risk |
|---|---|---|---|
| Direct Certificate Audit | Request ISO 13485:2023 certificate with specific scope covering your product category (e.g., “Class IIa Dental Imaging Devices”). Cross-check certificate number via IAF CertSearch. | Certificates without product scope, issued by non-accredited bodies (e.g., “China Certification & Inspection Group” ≠ CNAS-accredited), or >2 years old. | EU MDR Annex IX requires technical documentation audits by notified bodies. Suppliers without updated QMS face shipment rejection at EU ports. |
| CE Declaration Scrutiny | Demand full EU Declaration of Conformity listing: – Notified Body number (e.g., “0123”) – Applicable harmonized standards (e.g., EN ISO 7494-1:2022 for dental chairs) – Essential Requirements checklist |
Generic declarations without NB number, missing harmonized standards, or self-issued “CE” labels. | UKCA marking now required for UK market. Suppliers conflating CE/UKCA indicate regulatory illiteracy. |
| Factory Audit Trail | Verify unannounced audit records from NB. Request evidence of: – Biocompatibility testing (ISO 10993) – EMC reports (IEC 60601-1-2) – Sterilization validation (ISO 11135/11137) |
Refusal to share redacted audit reports, “generic” test certificates not tied to your PO. | China NMPA Class III requirements now mirror EU MDR. Suppliers without NMPA registration lack rigorous QA processes. |
Step 2: Negotiating MOQ (Strategic Volume Structuring)
Minimum Order Quantities (MOQs) directly impact inventory costs and market responsiveness. Leverage these technical negotiation levers:
| MOQ Strategy | Technical Rationale | Supplier Flexibility Indicator | 2026 Market Shift |
|---|---|---|---|
| Component-Based MOQ | Negotiate separate MOQs for: – Critical sub-assemblies (e.g., CBCT gantry: 5 units) – Consumables (e.g., sterilization trays: 50 units) Reduces capital lock-up for high-value items. |
Suppliers with modular production lines (e.g., separate clean rooms for electronics vs. mechanical assembly) offer tiered MOQs. | Post-2025, 73% of Chinese OEMs adopted Industry 4.0 systems enabling lot-size-1 production for certain components. |
| Phased Volume Commitment | Structure 12-month agreement with: – Q1: 30% of annual volume (validation) – Q2-Q4: 70% (scaling) Include quality KPIs (e.g., ≤0.5% field failure rate). |
Willingness to accept graduated volumes signals confidence in process stability and financial health. | Distributors now demand dynamic MOQs tied to real-time sales data via integrated ERP systems. |
| Sample Protocol | Insist on: – 3x pre-production samples with full test reports – Destructive testing rights – 15-day validation window |
Suppliers charging >150% of unit cost for samples often lack engineering capacity. | AI-powered sample validation (e.g., 3D scan comparison) now standard for premium suppliers. |
Step 3: Shipping Terms (Logistics Risk Allocation)
Incoterms® 2020 dictate cost/risk transfer points. For dental equipment, DDP (Delivered Duty Paid) is increasingly critical due to tariff volatility:
| Term | Risk Allocation | 2026 Cost Variables | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Risk transfers at ship rail. You bear: – Ocean freight volatility (±35% in 2025) – US Section 301 tariffs (7.5-25%) – Customs clearance delays |
Current spot rates: $4,200/40ft container Shanghai-LA. 2026 projections show 22% tariff uncertainty. | Only for distributors with in-house customs brokerage and freight insurance expertise. |
| DDP Your Clinic | Supplier manages: – All freight + insurance – Duty/tax calculation – Final-mile delivery You pay fixed landed cost |
Includes 2026 “green surcharge” (€150/container) and dynamic tariff management fees (~3.8% of CIF value). | Mandatory for clinics and distributors without logistics infrastructure. Eliminates hidden costs. |
Why Shanghai Carejoy Medical Co., LTD Exemplifies Best Practices
With 19 years of FDA/CE-compliant manufacturing (est. 2007), Shanghai Carejoy (Baoshan District, Shanghai) operationalizes this framework:
- Step 1 Verified: ISO 13485:2023 certificate #CN2025MD0017 (scope: dental chairs, CBCT, autoclaves), NB 0482 CE DoC with full EN ISO 7494-1:2022 validation
- Step 2 Optimized: Tiered MOQs (e.g., dental chairs: 1 unit for clinics, 5 units for distributors) with AI-driven production scheduling
- Step 3 Secured: DDP pricing to 45 countries via proprietary logistics platform (real-time customs duty calculator)
As a factory-direct OEM/ODM partner, Carejoy eliminates trading company markups while providing:
– Technical Documentation: Full MDR-compliant technical files
– Quality Assurance: In-line optical metrology for critical components
– Supply Chain Resilience: Dual-sourcing for motors/sensors (Shanghai + Singapore)
Strategic Partnership Inquiry
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 201900, China
Direct OEM/ODM Channel: [email protected]
Technical Procurement Hotline: +86 15951276160 (WhatsApp/WeChat)
Reference “2026 Sourcing Guide” for priority factory audit scheduling
Action Required: Request Carejoy’s 2026 Compliance Dossier (includes NB audit reports, DDP cost calculator, and modular MOQ matrix) before Q3 2025 supplier qualification.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Instruments in 2026
As dental technology evolves, procurement decisions must align with clinical performance, regulatory compliance, and long-term operational efficiency. Below are five critical FAQs for dental clinics and distributors evaluating dental instruments (instrument tandarts) in 2026.
| Question | Professional Insight |
|---|---|
| 1. What voltage requirements should I verify when importing dental instruments for use in Europe? | All dental instruments intended for use in EU member states must comply with the standard 230V ±10%, 50Hz electrical specifications. Verify CE marking and IEC 60601-1 certification for medical electrical equipment. Instruments sourced from non-EU markets (e.g., North America or Asia) may require voltage transformers or factory-configured dual-voltage models. Always confirm compatibility with local dental chair integration systems to prevent signal interference or motor degradation. |
| 2. How can I ensure long-term availability of spare parts for high-precision dental handpieces? | Partner only with OEMs or authorized distributors that guarantee spare parts availability for a minimum of 10 years post-discontinuation. Request a Spare Parts Lifecycle Agreement (SPLA) before purchase. Prioritize manufacturers with regional logistics hubs in Europe (e.g., Germany, Belgium) to reduce lead times. In 2026, modular handpiece designs with standardized bearings, gears, and O-rings are increasingly common—verify interchangeability and sterilization compatibility. |
| 3. Is professional installation required for electric dental motors and torque-controlled units? | Yes. Electric motors and advanced implantology units require certified technical installation to ensure calibration accuracy, vibration damping, and integration with the dental unit’s control software. Improper installation voids warranties and risks patient safety. Most leading brands (e.g., W&H, KaVo, NSK) offer white-glove installation services via trained field engineers. Distributors must coordinate site assessments for air pressure, water filtration, and power stability prior to delivery. |
| 4. What warranty terms are standard for dental instruments in 2026, and what do they cover? | The industry standard is a 2-year comprehensive warranty covering manufacturing defects, electronic components, and mechanical failure under normal use. Premium handpieces and motors may include extended 3–5 year options with predictive maintenance monitoring. Warranties exclude damage from improper sterilization, unauthorized repairs, or lack of preventive servicing. Always confirm whether the warranty is global or region-locked, especially for cross-border distribution. |
| 5. Are software updates and calibration included in the warranty or service plan? | In 2026, smart dental instruments with IoT connectivity (e.g., torque feedback, usage analytics) require regular firmware updates. These are typically included during the warranty period via secure cloud platforms or USB updates by certified technicians. Post-warranty, clinics must subscribe to a Service Assurance Plan (SAP) for continued software support. Calibration for torque accuracy and speed consistency should be performed annually and is often bundled in SAPs. |
Need a Quote for Instrument Tandarts?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


