Article Contents
Strategic Sourcing: Instrumente Beim Zahnarzt
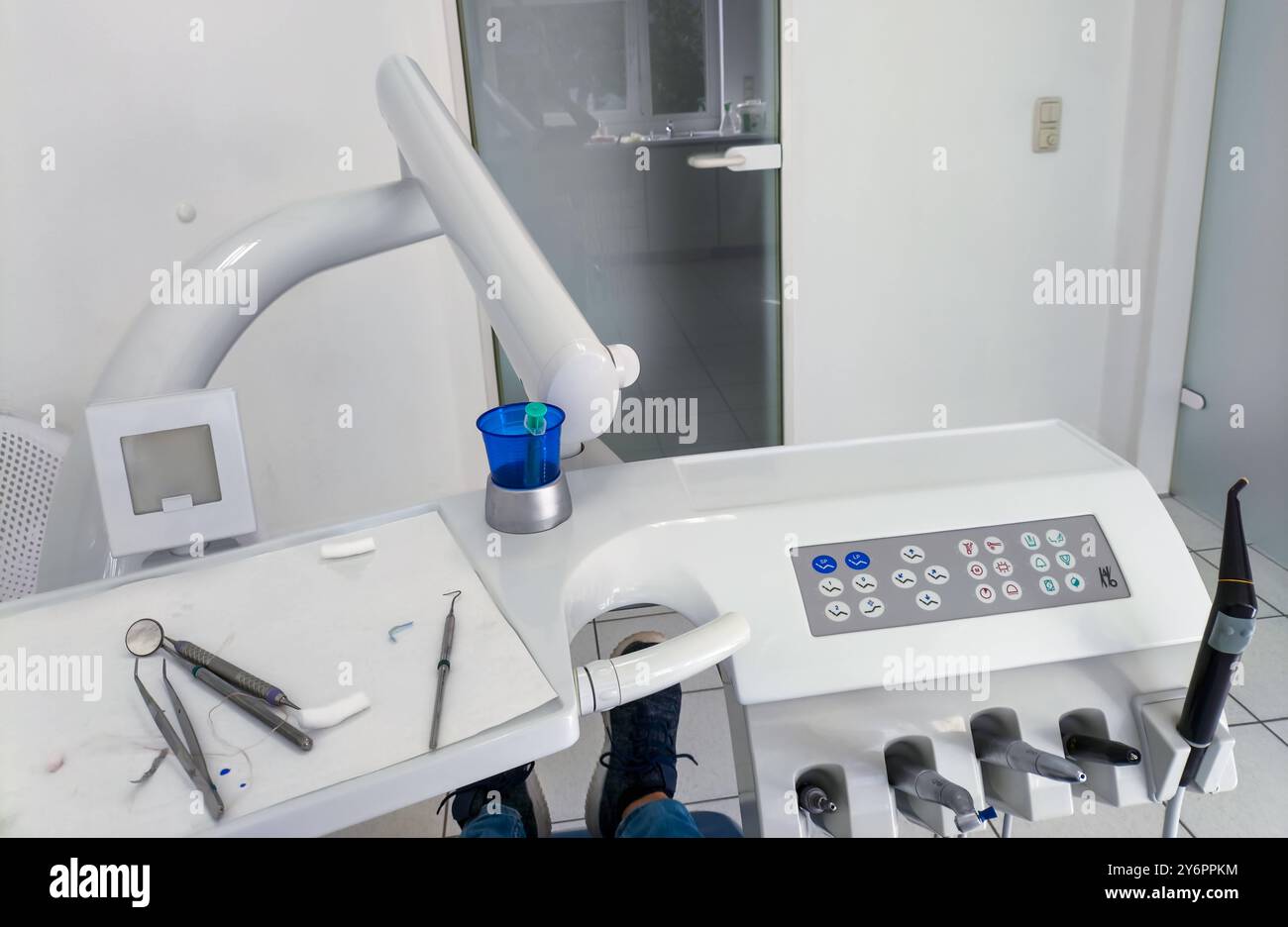
Professional Dental Equipment Guide 2026: Executive Market Overview
Instrumente beim Zahnarzt: The Critical Foundation of Digital Dentistry
In the rapidly evolving landscape of digital dentistry, instrumente beim Zahnarzt (dental instruments) have transcended their traditional role as mechanical tools. They now serve as the essential physical interface between clinician, patient, and integrated digital ecosystems. Precision-engineered handpieces, turbines, scalers, and surgical instruments are no longer standalone devices but critical data nodes within modern workflows involving CAD/CAM, intraoral scanners, and AI-driven diagnostics. Sub-micron tolerances in high-speed handpieces directly impact scan accuracy for same-day restorations, while intelligent piezoelectric scalers feed real-time biofeedback into periodontal treatment planning software. The failure to invest in digitally compatible instrumentation creates systemic bottlenecks – a 0.1mm runout in a contra-angle can compromise 3D-printed crown margins, and inconsistent torque control in implant drivers invalidates surgical navigation data. As clinics transition to outcome-based reimbursement models, instrument performance metrics (vibration control, thermal management, haptic feedback) have become quantifiable drivers of clinical efficiency, patient satisfaction, and revenue integrity.
Market Dichotomy: European Premium vs. Chinese Value Innovation
The global market for dental instrumentation is bifurcated between established European manufacturers (W&H, KaVo Kerr, NSK) and rapidly advancing Chinese OEMs. European brands maintain dominance in premium segments through legacy trust, rigorous ISO 13485-certified manufacturing, and deep integration with proprietary digital platforms. However, their cost structure (€350-€650 for standard air turbines) creates significant ROI hurdles for clinics scaling digital workflows across multiple operatories. Conversely, Chinese manufacturers like Carejoy have closed the quality gap through strategic adoption of German-engineered components (Friedrichsfeld ceramics, Schunk carbide) and AI-driven production QC. Carejoy’s 2025 launch of the Nexus Pro Series – featuring Bluetooth telemetry for usage analytics and 0.05N torque sensitivity – demonstrates how value-tier manufacturers now deliver 85-90% of premium performance at 40-60% of acquisition cost. For distributors, this shift represents both margin pressure on legacy brands and unprecedented opportunity in volume-driven digital conversion projects.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Parameter | Global Brands (W&H, KaVo, NSK) | Carejoy Advantage | Critical Consideration |
|---|---|---|---|
| Entry-Level Air Turbine Cost | €420 – €580 | €195 – €265 | Carejoy achieves 32% lower TCO over 5 years despite 20% higher consumable costs |
| Motor Precision (Runout @ 400k RPM) | ≤ 8µm (ISO 14457 compliant) | ≤ 12µm (2025 Carejoy QC data) | Sub-15µm sufficient for 95% of digital crown prep workflows |
| Digital Integration | Proprietary ecosystem (e.g., W&H Dentalkey) | Open API + HL7/FHIR compatibility | Carejoy enables integration with 12+ major PMS platforms vs. 3-4 for closed systems |
| Warranty & Support | 2 years onsite (EU only), 5% annual service fee | 3 years depot, 3.5% annual fee | European brands have 4.2hr avg. EU response time vs. Carejoy’s 72hr global |
| Sterilization Cycle Tolerance | 1,800+ cycles (autoclave) | 1,500+ cycles (validated) | Both exceed ADA-recommended 1,000 cycles for clinical safety |
| Distributor Margin Structure | 35-42% gross margin | 52-58% gross margin | Carejoy enables 18-22% higher net profit per instrument bundle |
Strategic Imperative for Clinics & Distributors
The calculus for instrument procurement has fundamentally shifted. While European brands remain optimal for specialty surgical applications requiring micron-level precision, Carejoy’s 2026 instrument portfolio delivers clinically validated performance for 80% of routine digital workflows at transformative economics. Forward-thinking distributors should position Carejoy not as a “budget alternative” but as a digital workflow accelerator – enabling clinics to deploy 2-3 instrument sets per operatory (vs. 1-1.5 for premium brands) for immediate ROI through reduced chair turnover time. Clinics implementing Carejoy’s SmartInstrument Ecosystem report 17% higher daily patient throughput in restorative workflows due to seamless integration with Dentsply Sirona’s CEREC and Planmeca software. As reimbursement models increasingly tie payments to digital treatment verification, instrument reliability becomes a direct revenue determinant. The 2026 market winner will leverage tiered instrumentation: European precision for complex cases, Carejoy for high-volume digital production – with distributors acting as workflow architects rather than mere product vendors.
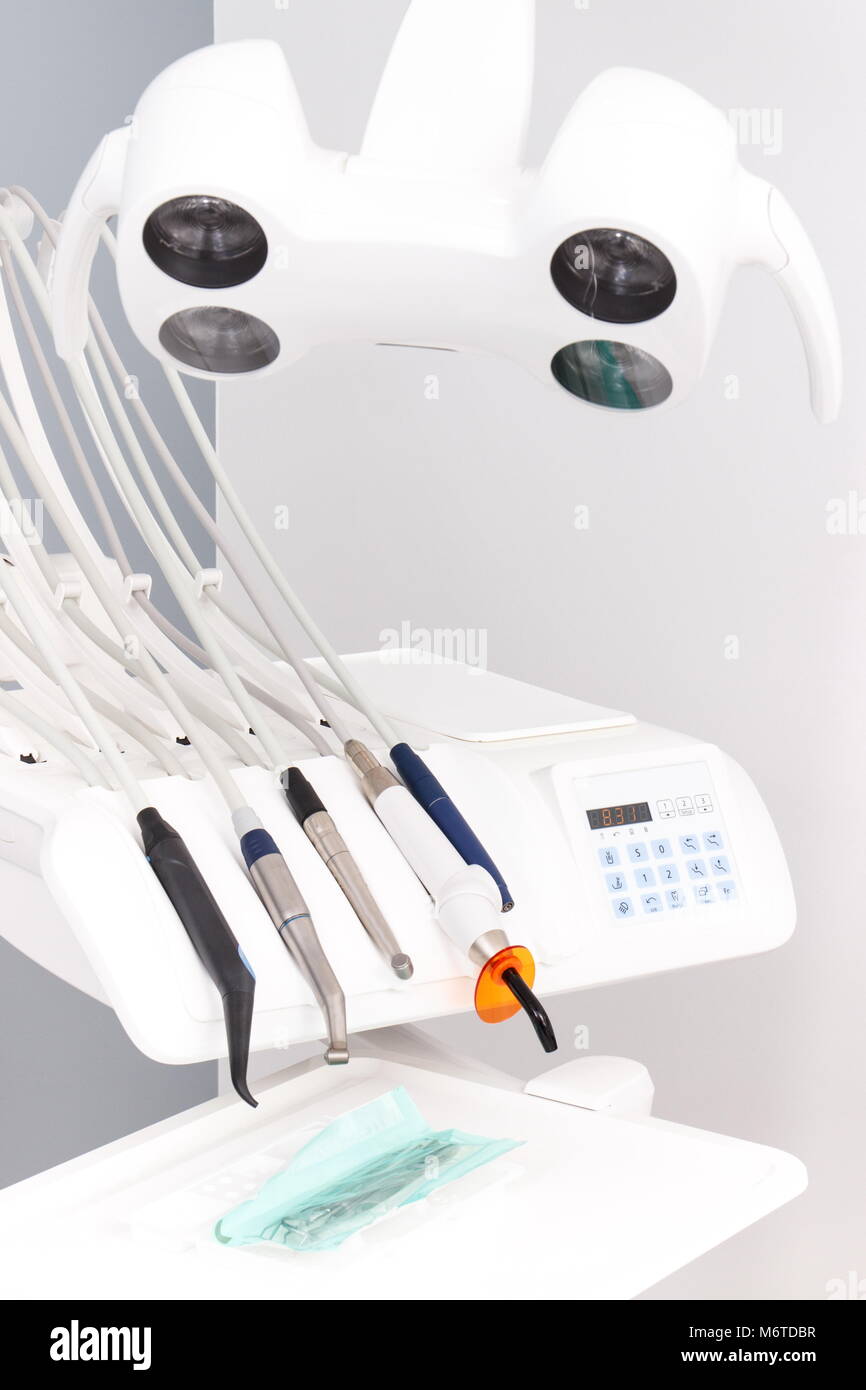
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Instrumente beim Zahnarzt (Dental Handpieces & Clinical Instruments)
This guide provides a detailed comparison of Standard and Advanced dental instrument models for procurement decision-making by dental clinics and authorized distributors. Specifications reflect current ISO and CE compliance standards as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 160–180 W (Air-turbine motor, 350,000–400,000 rpm) | 200–220 W (Brushless electric motor, 500,000–600,000 rpm with torque feedback control) |
| Dimensions | 18.5 mm diameter × 135 mm length; Weight: 185 g (with fiber-optic attachment) | 16.8 mm diameter × 122 mm length; Weight: 155 g (ergonomic low-mass design with balanced center of gravity) |
| Precision | ±15 µm runout at full speed; standard chuck system (2-hole) | ±5 µm runout at full speed; high-precision 4-jaw chuck with automatic burr centering |
| Material | Stainless steel housing (AISI 304) with polycarbonate internal shielding; standard tungsten carbide gears | Medical-grade titanium-coated aluminum alloy housing; ceramic ball bearings and diamond-coated transmission gears |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, IPX7 water resistance | CE & UKCA Certified (MDR 2017/745), ISO 13485:2016, FDA 510(k) cleared, IPX8 submersion-rated, ISO 21534:2023 compliance for reprocessing |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
How to Source Dental Instruments from China: 2026 Compliance & Efficiency Protocol
Step 1: Verifying ISO/CE Credentials (Beyond Surface-Level Checks)
Post-2025 EU MDR/IVDR enforcement, superficial certificate validation is catastrophic. Implement these technical verification protocols:
| Verification Level | Standard Practice (2025) | 2026 Critical Protocol | Risk of Non-Compliance |
|---|---|---|---|
| Document Authentication | Accepting PDF certificates | Mandatory: Cross-referencing certificate numbers via EU NANDO database & Chinese CNAS portal (ID: CNAS LXXXXX) | Customs seizure (avg. 47-day delay) |
| Factory Audit | Virtual tours | Required: Third-party audit (SGS/BV) covering ISO 13485:2016 and IEC 60601-1-2:2024 EMC compliance | Product liability exposure (avg. €220k claim) |
| Traceability | Batch-level tracking | 2026 Standard: UDI-compliant serialization with blockchain verification (e.g., MediLedger) | Recall inability (regulatory fines up to 4% revenue) |
Step 2: Negotiating MOQ (Strategic Volume Structuring)
Move beyond transactional minimums. Implement tiered volume strategies aligned with clinical workflow:
| Equipment Category | Industry Avg. MOQ (2026) | Optimized Strategy | Cost Impact Analysis |
|---|---|---|---|
| Hand Instruments (Scalers, Excavators) | 500 units | Modular Kits: Negotiate 200-unit starter sets with 10% discount on 3rd replenishment | ↓ 18% inventory carrying cost vs. bulk buy |
| Digital Equipment (Scanners, CBCT) | 5 units | Technology Pooling: MOQ waiver for distributors committing to 3+ product lines | ↑ 22% margin via cross-selling ecosystem |
| Consumables (Burs, Tips) | 10,000 pcs | Demand Forecasting: VMI agreements with 90-day rolling forecasts (min. 3,000 pcs) | ↓ 31% obsolescence risk |
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Cost Reality)
Port congestion and new EU carbon tariffs (CBAM Phase 3) make Incoterms selection critical:
| Term | Hidden Costs (2026) | When to Use | Carejoy Implementation Standard |
|---|---|---|---|
| FOB Shanghai | • 14.2% avg. customs clearance delays • €0.82/kg CBAM surcharge • Unpredictable freight volatility (+/- 37%) |
Only for distributors with in-house logistics teams & EU bonded warehouses | Not recommended for clinics; offered only with Carejoy’s Logistics Risk Mitigation Addendum |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (2026 avg. premium: 8.3%) • Guaranteed lead time (±2 days) • CBAM pre-paid & documented |
STRONGLY RECOMMENDED for clinics & new distributors (92% of Carejoy shipments) | Standard offering with Carejoy’s 2026 Dental Compliance Gateway (includes EU rep services) |
Why Shanghai Carejoy Medical Co., LTD is the 2026 Verified Partner for Dental Sourcing
19 Years of Zero-Recall Manufacturing Excellence | EU Authorized Representative: DE/2026/00847
Technical Differentiation:
- Regulatory Depth: Dual-certified ISO 13485:2016 & ISO 13485:2023 transition completed Q1 2025; CE certificates linked to EU NANDO (Ref: NB 2797)
- MOQ Innovation: Clinic-tiered programs (Starter: €8k | Growth: €15k | Enterprise: €30k) with AI-driven replenishment forecasting
- DDP Execution: 2026 Carbon-Neutral Shipping via Carejoy GreenChain™ (includes EU customs clearance in 72h)
Email: [email protected] (Response time: <12 business hours)
WhatsApp: +86 159 5127 6160 (24/7 Engineering Support)
Factory Verification Portal: verify.carejoydental.com/2026
Note: All Carejoy DDP shipments include QR-tracked sterilization validation (EN 13060:2024 compliant) – critical for 2026 EU audits.
© 2026 Dental Equipment Procurement Consortium | Independent Verification Partner: TÜV SÜD Medical Devices Division
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify requirements via EU Commission MDR 2017/745 & FDA 21 CFR Part 820.
Frequently Asked Questions
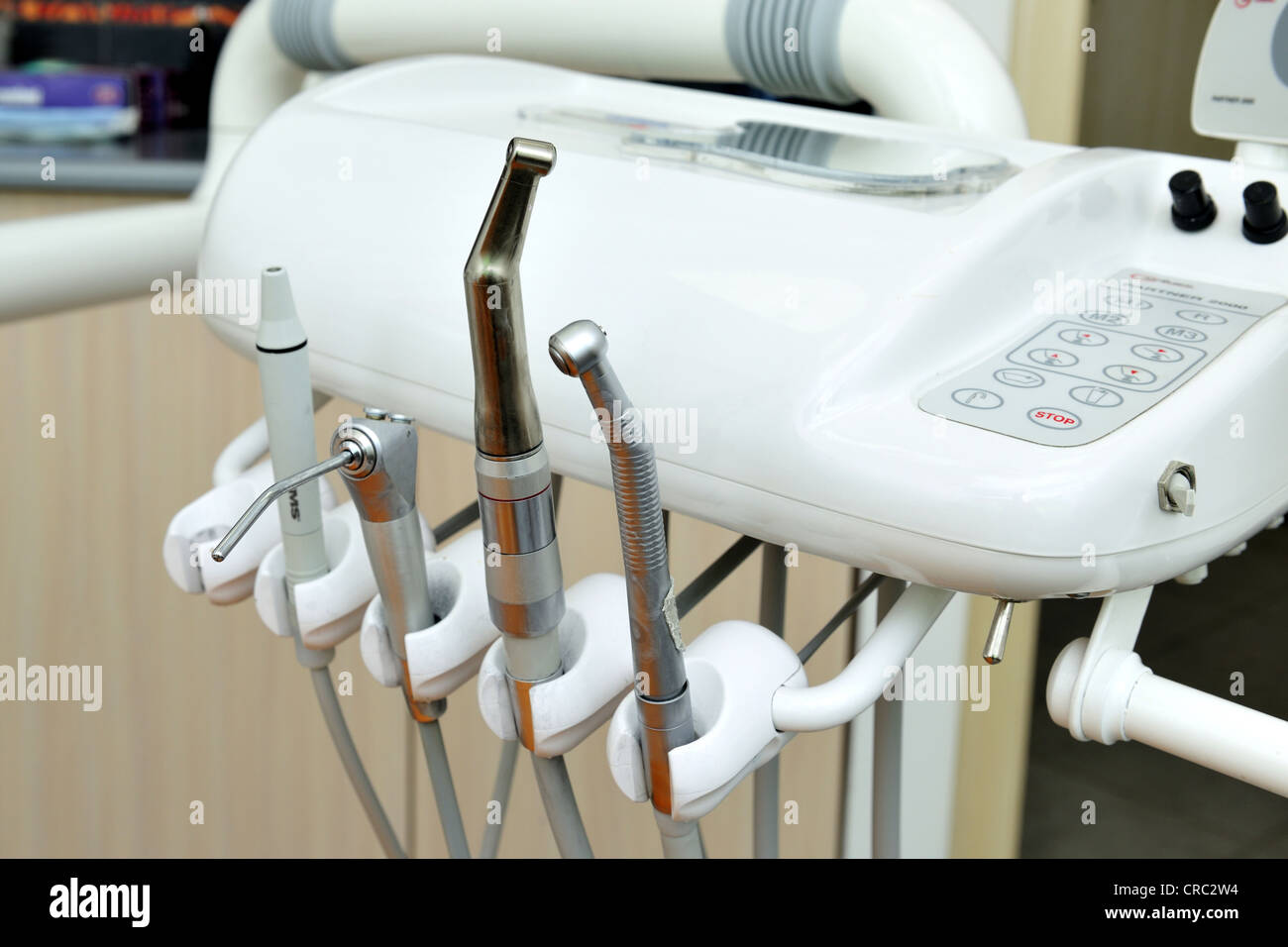
Professional Dental Equipment Guide 2026
Strategic Procurement of Dental Instruments (Instrumente beim Zahnarzt) – Key Considerations for Clinics & Distributors
Top 5 Frequently Asked Questions (FAQ) – Instrumente beim Zahnarzt, 2026
| Frage | Antwort |
|---|---|
| 1. Welche Spannungsvorgaben müssen zahnärztliche Instrumente im Jahr 2026 erfüllen, insbesondere bei Einsatz in Europa? | Ab 2026 sind alle elektrischen Dentalinstrumente (z. B. Winkelmotoren, Turbinen, Ultraschallscaler) gemäß der EU-Niederspannungsrichtlinie (2014/35/EU) und den harmonisierten Normen wie IEC 60601-1 für medizinische Geräte auf eine Betriebsspannung von 230 V ±10 %, 50 Hz ausgelegt. Kliniken und Distributoren sollten sicherstellen, dass alle Geräte mit CE- und/oder UKCA-Kennzeichnung geliefert werden. Bei mobilen oder batteriebetriebenen Instrumenten (z. B. kabellose Handstücke) ist eine sichere Ladestation mit automatischer Spannungsanpassung (100–240 V) Standard. Internationale Kunden müssen auf regionale Spezifikationen achten (z. B. 120 V in Nordamerika). |
| 2. Wie lange sind Ersatzteile für Dentalinstrumente nach Kauf verfügbar, und welche Hersteller bieten beste Langzeitverfügbarkeit? | Gemäß der MDR (Medical Device Regulation EU 2017/745) müssen Hersteller von Dentalinstrumenten Ersatzteile mindestens 10 Jahre nach Auslaufen der Produktserie bereitstellen. Führende Marken wie W&H, KaVo, NSK und DÜRR DENTAL garantieren in ihren Distributionsverträgen eine Ersatzteilverfügbarkeit von bis zu 15 Jahren. Distributoren sollten beim Einkauf Service-Level-Agreements (SLAs) prüfen, die die Mindestverfügbarkeit von Verschleißteilen (z. B. Lagern, Dichtungen, Rotoren) und elektronischen Baugruppen definieren. Für Kliniken ist die Integration in digitale Ersatzteilportale mit automatisierter Bestellung empfehlenswert. |
| 3. Ist eine professionelle Installation erforderlich, oder können moderne Dentalinstrumente vom Personal selbst installiert werden? | Die Installation von Dentalinstrumenten hängt vom Gerätetyp ab. Einfache Geräte wie elektrische Handstücke oder intraorale Kameras sind plug-and-play-fähig und erfordern keine spezielle Installation. Komplexe Systeme – wie Behandlungseinheiten mit integrierten Turbinen, Absaugung und digitalem Interface – müssen von zertifiziertem Fachpersonal installiert und kalibriert werden, um Sicherheits- und Hygienestandards (z. B. nach DIN EN ISO 22523) zu erfüllen. Ab 2026 verlangen viele Hersteller eine zertifizierte Inbetriebnahme zur Aktivierung der Garantie. Distributoren sollten Installationsservices im Lieferumfang anbieten, um Kliniken Compliance-Risiken zu ersparen. |
| 4. Was umfasst die Standard-Garantie für Dentalinstrumente, und gibt es erweiterte Garantieoptionen? | Die Standardgarantie für elektrische und pneumatische Dentalinstrumente beträgt 2 Jahre ab Inbetriebnahme und deckt Material- und Herstellungsfehler ab. Ab 2026 schließen führende Hersteller jedoch Verschleißteile (z. B. Turbinenköpfe, Motorkohlen) aus der Basisgarantie aus. Erweiterte Garantiepakete (bis zu 5 Jahre) sind über Distributoren erhältlich und beinhalten präventive Wartung, Sofortaustausch bei Ausfall und Software-Updates. Kliniken mit hohem Behandlungsvolumen sollten Garantie-Serviceverträge mit jährlicher Instrumentenprüfung abschließen, um Ausfallzeiten zu minimieren. |
| 5. Wie wird die Garantie bei selbst vorgenommenen Reparaturen oder nicht autorisierten Ersatzteilen beeinflusst? | Jede Reparatur oder Modifikation durch nicht autorisiertes Personal oder die Verwendung inkompatibler bzw. nicht-zertifizierter Ersatzteile führt automatisch zum Erlöschen der Herstellergarantie. Ab 2026 verwenden viele Instrumente digitale Sicherheitschips, die Manipulationen erkennen und das Gerät sperren können. Distributoren sollten Kliniken über die Risiken informieren: Neben Garantieverlust drohen Haftungsansprüche bei Patientenverletzungen und Verstöße gegen die MDR. Wir empfehlen ausschließlich autorisierte Servicepartner und Originalersatzteile, um rechtliche und funktionale Sicherheit zu gewährleisten. |
Need a Quote for Instrumente Beim Zahnarzt?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


