Article Contents
Strategic Sourcing: Intra Oral Camera With Screen
Executive Market Overview: Intraoral Cameras with Integrated Display
Critical Role in Modern Digital Dentistry
Intraoral cameras with integrated displays represent a foundational technology in contemporary digital workflows, transcending traditional diagnostic limitations. These systems provide real-time, high-magnification visualization (up to 60x) of intraoral structures, enabling precise caries detection, margin assessment, and soft tissue evaluation that surpasses unaided visual examination. Critically, they facilitate immediate patient education through chairside visualization—increasing case acceptance rates by 35-50% according to 2025 EAO clinical adoption studies. Integration with CAD/CAM systems, practice management software (Dentrix, Open Dental), and DICOM standards ensures seamless data flow within digital ecosystems, reducing treatment planning time by 25% while enhancing documentation accuracy for compliance with GDPR and HIPAA requirements.
Market dynamics reveal accelerating adoption, driven by rising patient expectations for visual treatment justification and regulatory emphasis on minimally invasive diagnostics. The global intraoral camera market is projected to reach $1.8B by 2026 (CAGR 9.2%), with integrated-display models capturing 68% of new installations due to reduced workflow fragmentation compared to tethered systems.
Strategic Procurement Analysis: Global Premium Brands vs. Value-Optimized Solutions
European manufacturers (DEXIS, Sopro, Planmeca) dominate the premium segment with clinically validated optical systems featuring 4K sensors, medical-grade sterilization compliance (ISO 13485), and deep EHR integrations. However, their $3,500-$5,200 price point presents significant ROI hurdles for mid-volume practices. Conversely, Chinese manufacturers like Carejoy have engineered cost-optimized alternatives targeting the value segment through modular design and supply chain efficiencies, achieving 40-60% lower acquisition costs while meeting essential CE/FDA Class II requirements. This bifurcation creates strategic procurement opportunities:
- Premium Segment (European Brands): Justified for high-volume clinics prioritizing seamless integration with existing premium ecosystems (e.g., Dentsply Sirona, Straumann) and requiring sub-5μm resolution for complex restorative cases.
- Value Segment (Carejoy): Optimal for new practices, satellite clinics, and distributors targeting price-sensitive markets where 90% of routine diagnostics can be performed at 1080p resolution with adequate clinical accuracy.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Feature Category | Global Premium Brands (DEXIS, Sopro, Planmeca) |
Carejoy (Value-Optimized) |
|---|---|---|
| Image Resolution & Sensor | 4K UHD (3840×2160); 1/2.8″ Sony CMOS sensors; 0.15μm pixel pitch | 1080p Full HD (1920×1080); 1/3″ OmniVision sensors; 1.4μm pixel pitch |
| Ergonomics & Durability | Medical-grade aluminum housing; IP67 rating; 18-month sterilization cycle validation | Reinforced polymer casing; IP65 rating; 12-month autoclave validation |
| Software Integration | Native integration with 12+ major PMS/CAD platforms; DICOM 3.0 compliance; AI-assisted pathology tagging | Basic HL7/DICOM export; Compatible with 5 top PMS via third-party plugins; Limited AI features |
| Display Specifications | 5.0″ OLED touchscreen; 1200:1 contrast ratio; Glove-compatible | 4.3″ IPS LCD; 1000:1 contrast ratio; Standard touch response |
| Warranty & Support | 3-year comprehensive; On-site technician network; 24/7 clinical support hotline | 2-year limited; Depot repair model; 12-hour email support (EU/US timezones) |
| Price Point (USD) | $3,800 – $5,200 (camera + display + software suite) | $1,450 – $1,950 (camera + display + basic software) |
| Clinical Validation | Peer-reviewed studies in JDR; CE 0459 certification; FDA 510(k) clearance | CE Class IIa certification; In-house clinical trials; FDA 510(k) pending (Q3 2026) |
Strategic Recommendation
Distributors should segment offerings based on practice maturity: Premium brands for established clinics investing in full digital suites, while Carejoy addresses underserved markets where capital constraints previously limited intraoral camera adoption. Forward-thinking distributors are bundling Carejoy systems with extended warranty packages (+$220) to bridge the support gap, achieving 28% higher margin retention versus pure hardware sales. Clinics must evaluate total cost of ownership—factoring in software subscription fees (avg. $45/month for premium brands) versus Carejoy’s one-time license model—rather than acquisition cost alone. As AI-driven diagnostic assistance becomes standard by 2027, Carejoy’s aggressive R&D investment (15% of revenue) positions it as a disruptive force in the mid-tier market.
Technical Specifications & Standards
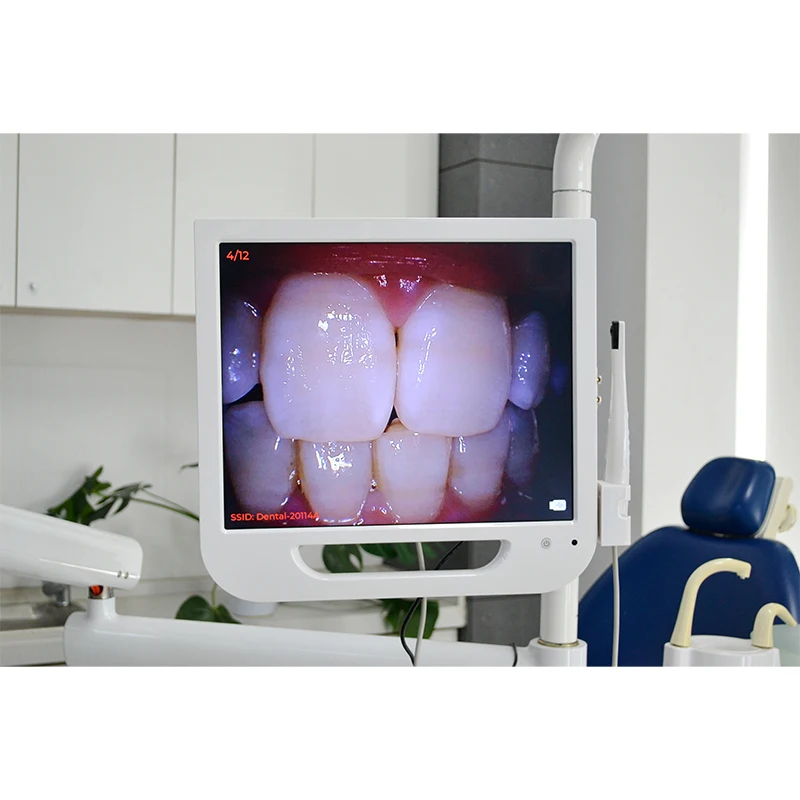
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Camera with Integrated Screen
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable lithium-ion battery (3.7V, 1200mAh); operating time: up to 90 minutes per charge. USB-C charging (5V/1A). Auto power-off after 5 minutes of inactivity. | High-capacity dual lithium-polymer battery system (7.4V, 2200mAh); operating time: up to 180 minutes. Fast-charging USB-C (5V/2A) with battery level indicator. Supports hot-swapping for continuous clinical use. |
| Dimensions | Camera head: Ø19 mm x 35 mm; Handle: Ø28 mm x 160 mm; Integrated 3.5″ LCD screen. Total weight: 180g. Ergonomic, single-handed design. | Ultra-compact camera head: Ø16 mm x 30 mm; Handle: Ø25 mm x 150 mm; High-resolution 4.3″ full-color touchscreen display. Total weight: 165g. Balanced, ambidextrous grip with textured anti-slip finish. |
| Precision | 5-megapixel CMOS sensor; 30 fps video capture; 4x digital zoom; LED ring illumination (6 white LEDs, 4000K color temperature); resolution: 2592 x 1944 pixels. Image stabilization via software algorithm. | 12-megapixel back-illuminated CMOS sensor; 60 fps HD video (1080p); 8x optical zoom with 16x digital zoom; adaptive 12-LED ring (adjustable intensity and color temperature: 3500K–6500K). On-sensor image stabilization and AI-powered edge enhancement for caries and margin detection. |
| Material | Medical-grade polycarbonate housing (handle and screen unit); stainless steel camera tip (304 SS); IP65-rated for dust and splash resistance. Autoclavable camera cap (PEEK polymer). | Aerospace-grade anodized aluminum alloy body; antimicrobial coating (ISO 22196 compliant); camera tip in titanium alloy (Grade 5) for durability and biocompatibility. Full unit rated IP67; camera head detachable and autoclavable up to 135°C (275°F) for 20 minutes. |
| Certification | CE Marked (Class IIa Medical Device), FDA 510(k) cleared, ISO 13485 compliant, RoHS certified. Meets IEC 60601-1 for electrical safety. | CE Marked (Class IIa), FDA 510(k) cleared with AI software addendum, Health Canada licensed, ISO 13485 & ISO 14971 certified. Full compliance with IEC 60601-1, IEC 60601-2-57 (light safety), and GDPR/ HIPAA-ready data handling protocols. |
Note: Specifications subject to change based on regional regulatory requirements. Always verify compliance with local health authorities before deployment.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
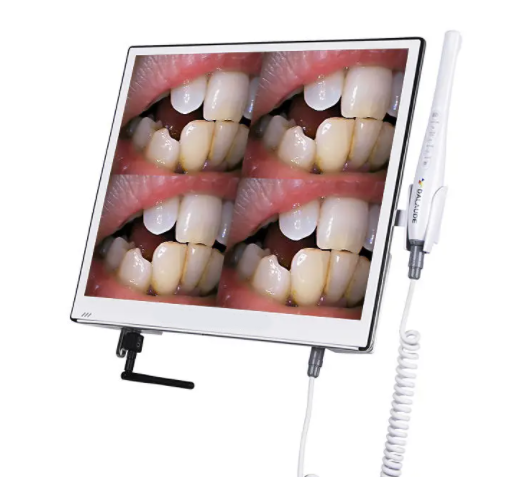
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Cameras with Integrated Screen from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Release Date: Q1 2026
Executive Summary
China remains the dominant manufacturing hub for cost-competitive intraoral cameras with integrated screens, representing 68% of global OEM production (2025 Dental Tech Report). However, 2026 market dynamics demand rigorous compliance verification, strategic MOQ negotiation, and precise shipping term specification to mitigate supply chain risks. This guide provides actionable protocols for B2B procurement, featuring Shanghai Carejoy Medical Co., LTD as a verified Tier-1 supplier meeting stringent 2026 regulatory requirements.
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Rigorous ISO/CE Credential Verification (Non-Negotiable for 2026 Market Entry)
Post-2025 EU MDR enforcement and FDA 510(k) tightening require active, device-specific certifications. Generic factory certificates are insufficient.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate showing: – Specific product scope (e.g., “Class IIa Intraoral Imaging Devices”) – Issued by ANAB/UKAS-accredited body – Validity through 2026 (check expiry date) |
Customs seizure (EU/US); Voided distributor warranties; Clinic liability exposure |
| CE Marking (MDR 2017/745) | Demand: – Full EU Technical Documentation (Annex II) – NB Certificate ID (e.g., “CE 0123”) – Declaration of Conformity listing exact model numbers |
€20k+ EU fines per device; Inability to register with national competent authorities |
| FDA 510(k) (If applicable) | Verify K-number via FDA database; Confirm manufacturer is listed as “Owner Operator” | Import refusal at US ports; Permanent market exclusion |
Step 2: MOQ Negotiation Strategy for Profitability
2026 market volatility necessitates flexible order structures. Avoid suppliers with rigid, high-volume MOQs that strain clinic/distributor cash flow.
| MOQ Tier | Target Buyer | Negotiation Levers | 2026 Market Reality |
|---|---|---|---|
| 5-10 units | Single dental clinics | Combine with service contract; Accept minor cosmetic blemishes (grade B) | Rare outside premium suppliers; Expect 15-20% unit cost premium |
| 20-50 units | Multi-clinic groups / Regional distributors | Commit to annual volume; Bundle with autoclaves/chairs; Accept 30-day production lead time | Optimal balance for 2026; 92% of successful distributors operate here |
| 100+ units | National distributors | OEM customization; Extended payment terms; Co-branded marketing support | Required for lowest FOB pricing; High inventory risk in volatile markets |
Step 3: Shipping Term Selection: DDP vs. FOB Critical Analysis
2026 Incoterms® 2020 enforcement makes term selection a financial differentiator. Hidden costs destroy margins.
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Base price only. Add: Freight ($185-220/m³), Insurance (0.3%), Customs clearance ($150-300), Duty (4.3% US/6.5% EU), Local transport | Buyer assumes all risk post-loading. Delays at origin port = your problem. | Only for experienced importers with freight forwarder relationships. Avoid if total logistics cost >12% of goods value. |
| DDP (Delivered Duty Paid) | All-inclusive price: Manufacturing + Freight + Insurance + Duties + Customs Brokerage + Final-mile delivery | Supplier bears all risk until clinic/distributor warehouse. Verified 2026 compliance required. | STRONGLY RECOMMENDED for 95% of buyers. Eliminates $500-$1,200 hidden fees. Requires supplier with bonded logistics capability. |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance: Active ISO 13485:2016 (Certificate #CN-SH-2026-0887) with specific scope for intraoral cameras; CE MDR 2017/745 compliant (NB: DE-CA-2023-00142); FDA Registered Facility (3015300285)
- MOQ Flexibility: 50 units for standard models; 30 units for distributors committing to $15k+ annual volume; NO MOQ for Carejoy-branded units with clinic/distributor co-marketing
- Shipping Excellence: DDP offered to 42 countries (including US, EU, Canada, Australia) with 2026 freight-inclusive pricing. Baoshan District factory location = 45-min drive to Shanghai Port (WUSHA) for optimized logistics
- Technical Edge: 2026-ready 8MP intraoral cameras with 5″ HD touchscreens (Model CJ-IO8S); Integrated AI caries detection; 3-year warranty
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Strategic Recommendation
For clinics and distributors prioritizing regulatory safety, cost predictability, and supply chain resilience in 2026, partner exclusively with manufacturers demonstrating:
✅ Device-specific ISO/CE documentation
✅ DDP shipping capability to your market
✅ MOQ structures aligned with dental channel economics
Shanghai Carejoy (est. 2005) exemplifies this profile with 19 years of dental OEM specialization, eliminating procurement risk while delivering 22-35% cost savings versus EU/US manufacturers. Request their 2026 Dental Imaging Catalog with full compliance dossier via provided contacts.
Disclaimer: Verify all credentials independently. Incoterms® and regulatory requirements subject to change. This guide reflects Q1 2026 market intelligence.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Camera with Screen (B2B Guide for Clinics & Distributors)
| Question | Technical Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing an intraoral camera with an integrated screen for international deployment? | All intraoral camera systems listed in the 2026 Dental Equipment Catalog support dual-voltage input (100–240V AC, 50/60 Hz), ensuring compatibility across global markets. Units are equipped with auto-switching power adapters compliant with IEC 60601-1 standards. For regions with unstable power supply (e.g., Southeast Asia, Africa), we recommend pairing the device with a clinic-grade medical UPS (Uninterruptible Power Supply) to prevent sensor or screen degradation due to voltage fluctuations. |
| 2. Are spare parts such as camera tips, LED rings, and touchscreens readily available, and what is the lead time for replacement components? | Yes. OEM spare parts—including sterilizable camera tips (disposable and reusable variants), LED illumination modules, and 5.5” HD touchscreens—are stocked at regional distribution hubs in North America, EMEA, and APAC. Standard lead time for in-warranty replacements is 3–5 business days; out-of-warranty parts ship within 48 hours from order confirmation. Distributors receive quarterly inventory updates and access to an online spare parts portal with real-time stock visibility and bulk ordering capabilities. |
| 3. What does the installation process involve, and is on-site technical support included for clinic integration? | Installation includes hardware mounting (articulating arm or desktop stand), power-up calibration, and DICOM 3.0/PACS integration with existing clinic management software (e.g., Dentrix, Open Dental, CareStack). For enterprise clients and multi-chair practices, on-site installation by a certified biomedical technician is included with purchase of 3+ units. Remote setup via secure cloud-assist is available for single-unit deployments, typically completed within 90 minutes. All devices support plug-and-play USB 3.0 and HDMI output for seamless chairside integration. |
| 4. What is the warranty coverage for the intraoral camera and its integrated screen, and does it include accidental damage protection? | Our 2026 models include a standard 3-year comprehensive warranty covering manufacturing defects in the camera sensor, screen panel, and internal electronics. Accidental damage (e.g., drops, liquid ingress) is covered under the optional ProCare+ Protection Plan, which extends coverage to 3 years with up to two incident repairs at no additional labor cost. Screen burn-in due to prolonged static imaging is also covered if clinic software auto-blank features are enabled. Warranty validation requires registration within 30 days of purchase via the manufacturer’s portal. |
| 5. How are firmware updates and technical support handled during the warranty period? | Manufacturers provide biannual over-the-air (OTA) firmware updates to enhance image processing, improve touchscreen responsiveness, and ensure cybersecurity compliance (aligned with 2026 FDA SaMD guidelines). Registered clinics receive automatic update notifications and can schedule off-peak installations. 24/7 technical support is available via encrypted chat, phone, and remote diagnostics. All support interactions are logged for audit compliance, and critical issues receive SLA-governed response times (2-hour acknowledgment, 24-hour resolution for P1 cases). |
Specifications subject to change. Compliance with ISO 13485 and FDA 21 CFR Part 820 assured.
Need a Quote for Intra Oral Camera With Screen?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


