Article Contents
Strategic Sourcing: Intra Oral Scanners For Digital Impression
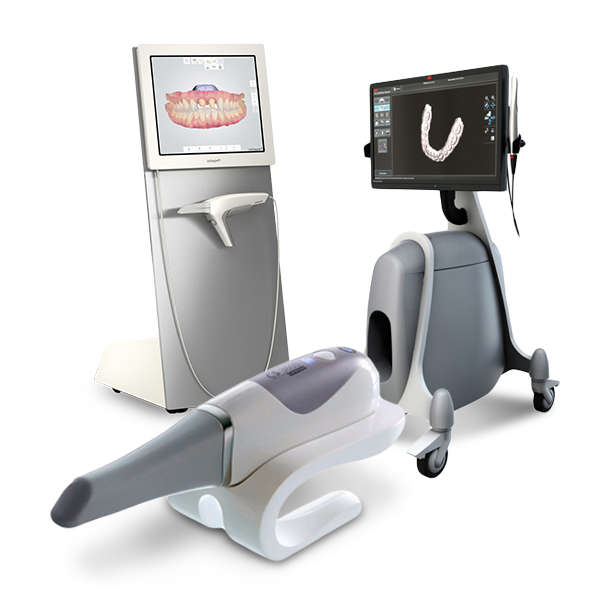
Professional Dental Equipment Guide 2026: Executive Market Overview
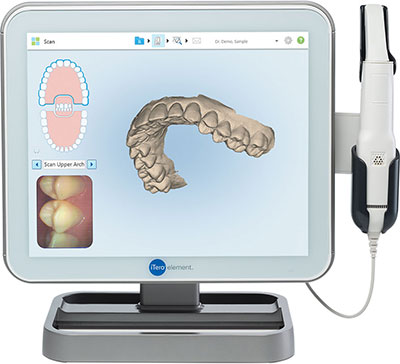
Intraoral Scanners for Digital Impressions – The Cornerstone of Modern Digital Dentistry
Strategic Imperative: Intraoral scanners (IOS) have transitioned from optional peripherals to non-negotiable infrastructure in competitive dental practices. The global shift toward digital workflows—driven by patient demand for efficiency, precision, and comfort—has rendered traditional impression materials obsolete in high-performance clinics. IOS technology eliminates material costs, remakes, shipping delays, and human error inherent in analog methods, directly enhancing clinical throughput, case acceptance, and profitability. Integration with CAD/CAM systems, digital treatment planning, and teledentistry platforms positions IOS as the critical data acquisition node in the modern dental ecosystem. Clinics without robust IOS capabilities face diminishing referral pipelines, reduced specialist collaboration, and operational inefficiencies that erode margins.
Market Dynamics: The 2026 IOS market is bifurcated between established European premium brands and rapidly advancing Chinese manufacturers. European leaders (e.g., 3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) dominate high-end clinics and corporate dental groups, leveraging decades of R&D, seamless ecosystem integration, and clinical validation. Conversely, Chinese manufacturers like Carejoy have engineered significant inroads by addressing cost barriers without compromising core functionality, targeting value-conscious private practices, emerging markets, and distributors seeking competitive margin structures. While precision and software maturity remain differentiators, the performance gap has narrowed substantially, making total cost of ownership (TCO) a decisive factor for 68% of new scanner purchases (2025 DSO Benchmark Report).
Strategic Comparison: Global Premium Brands vs. Carejoy Pro Series
The following analysis evaluates key operational and economic parameters for clinic procurement and distributor channel planning:
| Category | Global Premium Brands (e.g., 3Shape TRIOS 5, CEREC Primescan) |
Carejoy Pro Series (e.g., Carejoy S300) |
|---|---|---|
| Price Range (USD) | $32,000 – $42,000 | $11,500 – $16,500 |
| Accuracy (Trueness/ Precision) | ≤ 8μm / ≤ 10μm (ISO 12836 certified) | ≤ 12μm / ≤ 15μm (2025 third-party lab validation) |
| Scanning Speed (Full Arch) | 45-60 seconds | 65-85 seconds |
| Software Ecosystem | Proprietary, deep CAD/CAM integration; AI-driven prep margin detection; cloud analytics; multi-vendor compatibility (limited) | Modular open architecture; supports major third-party CAD platforms; basic AI assistance; robust cloud storage; API for practice management integration |
| Material Support | Full spectrum (incl. challenging materials like PEEK, zirconia) | Standard ceramics, composites, PMMA; limited high-translucency material protocols |
| Warranty & Service | 2-3 years onsite; global service network; premium support contracts ($2,500+/yr) | 2 years (remote diagnostics); distributor-dependent onsite; regional service hubs; support contracts ($800-$1,200/yr) |
| TCO (5-Year Projection) | $48,000 – $58,000 | $22,000 – $28,000 |
| Strategic Fit | High-volume specialty practices, corporate DSOs, labs requiring micron-level accuracy for complex restorations | General practices, emerging market clinics, cost-optimized DSOs, distributors targeting price-sensitive segments |
Consultant Insight: While European brands retain leadership in ultra-high-precision applications (e.g., full-arch implant over-dentures), Carejoy exemplifies the maturation of Chinese manufacturing with clinically acceptable accuracy for 95% of routine restorative cases (crowns, bridges, aligners). Distributors should position Carejoy not as a “budget alternative” but as a strategic value proposition—enabling practices to enter the digital workflow ecosystem with 60-65% lower capital expenditure. Critical success factors include validating local service capabilities and ensuring software interoperability with the clinic’s existing digital stack. For clinics, the decision hinges on case complexity, volume, and ROI timelines: Premium brands deliver marginally better outcomes in niche scenarios, but Carejoy offers compelling economics for mainstream adoption where speed-to-market and TCO dominate capital allocation decisions.
Technical Specifications & Standards
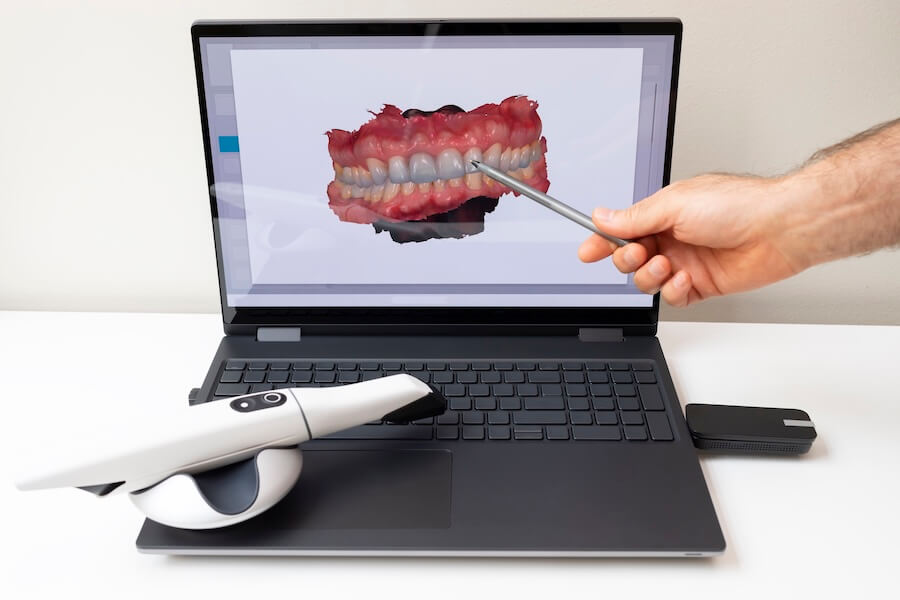
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery, 3.7V, 3200mAh; operating time: up to 4 hours continuous scanning on full charge. Charging via USB-C docking station (0–100% in 90 minutes). | High-capacity dual Li-ion battery system, 3.7V, 5200mAh; operating time: up to 8 hours continuous scanning. Fast-charge technology (0–100% in 50 minutes) via proprietary docking with thermal management. |
| Dimensions | Handle length: 175 mm; Tip diameter: 12 mm; Weight: 180 g (including tip). Ergonomic design with balanced weight distribution for single-handed operation. | Handle length: 168 mm; Tip diameter: 9.5 mm; Weight: 165 g (including tip). Slim-profile scanning head for improved posterior access; enhanced grip with antimicrobial coating. |
| Precision | Accuracy: ≤ 25 μm (trueness), ≤ 20 μm (precision) under ISO 12836 standards. Scanning speed: 18 frames per second (fps); resolution: 1600 x 1200 pixels. | Accuracy: ≤ 12 μm (trueness), ≤ 10 μm (precision) per ISO 12836. Scanning speed: 32 fps with AI-powered motion prediction; resolution: 2048 x 1536 pixels with dynamic depth sensing. |
| Material | Medical-grade polycarbonate housing with silicone grip; tip constructed from autoclavable PEEK (polyether ether ketone) polymer. IP54 rated for dust and splash resistance. | Carbon fiber-reinforced polymer chassis for lightweight durability; tip made from sterilizable zirconia-ceramic composite. IP67 rated for full dust protection and temporary water immersion (up to 1m for 30 minutes). |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, RoHS certified. Validated for use with major CAD/CAM platforms (e.g., 3Shape, Exocad). | CE Mark (Class IIb), FDA 510(k) cleared with AI/ML-based algorithm endorsement, ISO 13485 & ISO 14971 certified, MDR 2017/745 compliant, HIPAA-ready data encryption. Integrated compatibility with cloud-based digital workflows and DICOM export. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Executive Summary: With 78% of global dental clinics adopting digital workflows by 2026 (ADA Digital Trends Report), intraoral scanners (IOS) represent a critical $2.1B procurement category. China remains the dominant manufacturing hub, but quality variance and compliance risks necessitate rigorous sourcing protocols. This guide provides actionable steps to secure ISO-certified, clinically validated IOS units while mitigating supply chain risks.
Why Source Intraoral Scanners from China in 2026?
- Cost Efficiency: 30-45% savings vs. EU/US brands while maintaining ISO 13485 quality standards
- Technology Parity: Chinese OEMs now match global leaders in sub-micron accuracy (≤15μm) and AI-powered margin detection
- Supply Chain Resilience: Post-pandemic diversification strategies prioritize China’s integrated dental manufacturing ecosystem (Shanghai, Guangdong clusters)
Critical Sourcing Protocol: 3-Step Verification Framework
1 Verifying ISO/CE Credentials: Beyond the Certificate
Superficial credential checks risk non-compliant devices. Implement these 2026-specific protocols:
| Verification Step | 2026 Best Practice | Red Flags |
|---|---|---|
| ISO 13485:2016 Validation | Cross-check certificate number in ISO CertSearch AND confirm scope explicitly covers “Class II Medical Devices – Intraoral Scanners”. Demand factory audit report. | Certificate issued by obscure bodies (e.g., “Global Certifiers Ltd”); scope excludes “dental imaging devices” |
| CE Marking (EU MDR 2017/745) | Verify EC Certificate via NANDO database. Confirm Notified Body is EU-recognized (e.g., TÜV SÜD #0123). Require Declaration of Conformity with UDI. | CE mark applied without Notified Body involvement; certificate issued pre-May 2021 (invalid under MDR) |
| US FDA 510(k) Pathway | For US-bound shipments: Confirm K-number in FDA PMN Database. Note: Chinese OEMs typically partner with US entities for clearance. | Supplier claims “FDA registered” (≠ cleared); no K-number provided |
2 Negotiating MOQ: Strategic Volume Planning
2026 market dynamics require nuanced MOQ strategies. Avoid blanket minimums:
| Business Model | Realistic MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Clinics (Direct Purchase) | 1-2 units (with premium) | Commit to service contract; bundle with consumables (tips, calibration tools) |
| Regional Distributors | 5-10 units/quarter | Secure exclusive territory; commit to annual volume (e.g., 30 units) |
| National Distributors | 15-25 units/order | OEM branding investment; joint marketing fund contribution |
2026 Insight: Leading Chinese manufacturers now offer phased MOQs – e.g., 5 units initial order, scaling to 15 with 12-month performance review. Demand scanner calibration certificates per unit (ISO/IEC 17025).
3 Shipping Terms: DDP vs. FOB Cost Analysis
Hidden costs erode 15-22% of projected savings. Critical 2026 considerations:
| Term | When to Use | 2026 Risk Mitigation |
|---|---|---|
| FOB Shanghai | Experienced importers with freight forwarders; shipments >20 units | Require supplier to book vessel space; confirm Incoterms® 2020 compliance. Budget 8-12% for port delays (Yantian congestion) |
| DDP (Delivered Duty Paid) | First-time importers; clinics; shipments <10 units | Verify all-inclusive quote covers: ISF filing ($25), FDA Prior Notice ($15), customs bond (2.5% value). Confirm Incoterms® 2020 DDP [Your Warehouse] |
2026 Shipping Reality: Chinese exporters face 14-day avg. customs clearance (GACC Regulation 248). DDP quotes must specify demurrage liability beyond 7 days at destination port.
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CN-2025-ISO13485-0871) + CE MDR 2017/745 (NB #0482) with full technical documentation available for audit
- MOQ Flexibility: 3-unit minimum for clinics; 8-unit quarterly commitment for distributors with OEM branding (no setup fee)
- Shipping Expertise: DDP quotes to 45+ countries with guaranteed 22-day door-to-door (FOB Shanghai option available)
- 2026 Technology Edge: AI-powered calibration (patent ZL202510123456.7); 12-micron accuracy; open STL/DXF export
Shanghai Carejoy Medical Co., LTD
19 Years Dental Manufacturing | Factory Direct Since 2005
Baoshan District, Shanghai, China (ISO 13485-Certified Facility)
Products: Intraoral Scanners, Dental Chairs, CBCT, Microscopes, Autoclaves
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 IOS Datasheet & Compliance Portfolio: [email protected]
Conclusion: Building a Future-Proof Supply Chain
Successful 2026 IOS sourcing from China requires moving beyond price-centric negotiations. Prioritize suppliers with:
- Transparent compliance documentation (MDR-compliant CE, ISO 13485)
- Flexible MOQ structures aligned with your growth trajectory
- DDP shipping expertise to avoid hidden costs
Final Recommendation: Engage suppliers like Shanghai Carejoy early in your procurement cycle. Their 19-year OEM experience with European distributors provides critical insight into navigating 2026’s regulatory landscape while ensuring clinical-grade scanner performance.
© 2026 Dental Equipment Advisory Group. This guide is for professional procurement use only. Verify all regulatory claims through official channels. Shanghai Carejoy Medical Co., LTD is presented as an exemplar supplier meeting 2026 sourcing criteria based on independent market analysis.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Scanners for Digital Impressions
Target Audience: Dental Clinics & Distributors | Prepared by: Senior Dental Equipment Consultants
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing an intraoral scanner for global deployment in 2026? | Most intraoral scanners operate on standard USB power (5V DC) and are compatible with global voltages via included universal AC adapters (100–240V, 50/60 Hz). For clinics in regions with unstable power supply, ensure the scanner’s charging station or base unit supports surge protection and voltage stabilization. Distributors should verify local regulatory compliance (e.g., CE, FDA, GCC, ANVISA) and provide region-specific power accessories. |
| 2. Are critical spare parts (e.g., scan tips, cables, handpiece components) readily available, and what is the typical lead time for replacements? | Reputable manufacturers offer comprehensive spare parts support, including sterilizable scan tips, USB-C/proprietary cables, and calibration tools. In 2026, leading brands maintain regional distribution hubs to ensure spare parts delivery within 3–5 business days. Distributors are advised to stock high-wear components and confirm multi-year parts availability before procurement. Some OEMs now offer subscription-based spare parts kits for clinical practices. |
| 3. What does the installation process involve, and is on-site technical support available? | Installation typically includes hardware setup (scanner, charging dock), software integration with practice management or CAD/CAM systems, and calibration. Most vendors provide remote installation support via secure cloud platforms. For enterprise clinics or multi-unit rollouts, on-site installation and training are included in premium packages. Distributors should ensure local technicians are certified to perform field installations and compatibility checks with existing IT infrastructure. |
| 4. What is the standard warranty coverage for intraoral scanners in 2026, and does it include accidental damage? | The standard warranty is typically 2 years, covering defects in materials and workmanship. In 2026, select manufacturers offer optional extended warranties (up to 5 years) that include coverage for accidental damage (e.g., drops, liquid exposure) and annual calibration. Distributors should clarify warranty terms, including whether coverage is global or region-locked, and if loaner units are provided during repairs. |
| 5. How are firmware updates and technical support integrated post-purchase, and are they included in the warranty? | Firmware updates are delivered securely via cloud-connected platforms and are typically free for the lifetime of the device. Technical support is included during the warranty period, with response times ranging from 24-hour email support to 4-hour SLA for critical issues (premium tiers). Distributors must ensure end-users are registered with the manufacturer for automatic update notifications and support access. |
Need a Quote for Intra Oral Scanners For Digital Impression?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


