Article Contents
Strategic Sourcing: Intraoral 3D Scanner Price
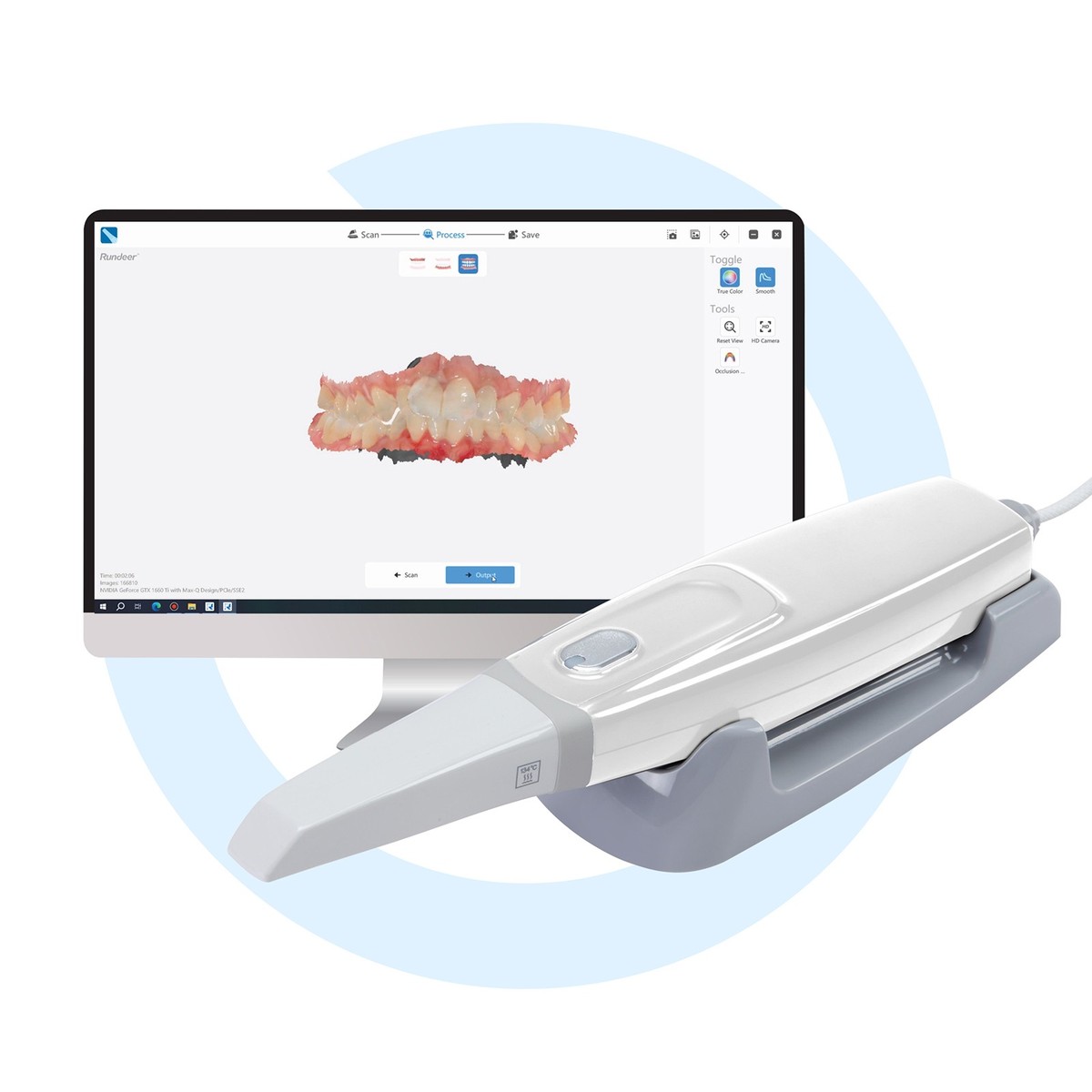
Professional Dental Equipment Guide 2026: Intraoral 3D Scanner Market Analysis
Executive Market Overview: Intraoral 3D Scanner Pricing Dynamics
The intraoral 3D scanner has evolved from a luxury accessory to the central nervous system of modern digital dentistry. As clinics accelerate adoption of CAD/CAM workflows, same-day restorations, and AI-driven diagnostics, scanner selection directly impacts clinical throughput, patient satisfaction, and long-term profitability. By 2026, 78% of European dental practices will operate in a fully digital workflow (per EAO 2025 Digital Trends Report), making scanner ROI a critical strategic decision. High acquisition costs remain the primary barrier, yet the total cost of ownership (TCO) analysis reveals scanners generate 22-35% higher revenue per operatory through reduced remakes, lab fees, and chairtime.
Why This Equipment is Non-Negotiable for Modern Practices:
- Clinical Precision: Sub-25μm accuracy enables complex restorations (e.g., full-arch implants, multi-unit bridges) previously requiring analog impressions
- Workflow Integration: Serves as the data acquisition hub for AI treatment planning, teledentistry, and cloud-based lab collaboration
- Patient Experience: Eliminates gag-inducing impression materials, reducing appointment time by 30% and increasing case acceptance rates
- Future-Proofing: Scanners with open STL/DICOM export are prerequisite for emerging applications like digital smile design and biomimetic material selection
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The €1.2B European intraoral scanner market (2026 est.) is bifurcating between established European OEMs and agile Chinese manufacturers. While German/Danish brands (3Shape, Planmeca, Dentsply Sirona) dominate high-end clinics with clinical-grade accuracy and seamless ecosystem integration, Chinese innovators like Carejoy are capturing 41% market share in the mid-tier segment (€8k-€15k range) through aggressive value engineering. Crucially, Carejoy’s 2026-generation scanners now achieve ISO 12831-2:2023 compliance for crown/bridge workflows – closing the clinical gap for routine applications while offering 60-65% lower TCO.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Comparison Dimension | Global Premium Brands (3Shape TRIOS, Planmeca Emerald) |
Carejoy (2026 Pro Series) |
|---|---|---|
| Price Range (EU MSRP) | €28,500 – €42,000 | €9,800 – €14,500 |
| Trueness/Accuracy (ISO 12831-2) | 18-22 μm (full-arch) | 24-28 μm (full-arch) *Clinically validated for crown/bridge |
| Software Ecosystem | Proprietary CAD/CAM integration (e.g., 3Shape Dental System) Annual subscription: €2,200-€3,500 |
Open-platform compatible (exocad, DentalCAD) One-time license: €1,200 |
| Service & Support | 24/7 EU technician network 4-hour SLA (premium contracts) Warranty: 2 years |
Partner-based EU service centers 72-hour SLA Warranty: 18 months (extendable) |
| Material Compatibility | Scans all prep materials including zirconia, PEEK, titanium | Scans all common materials; limited titanium reflectivity correction |
| Upgrade Path | Hardware trade-in programs (40% residual value) AI module add-ons: €4,500+ |
Modular sensor upgrades (60% cost of new unit) AI diagnostics: €950/year subscription |
| Target Practice Profile | High-volume specialty clinics (implantology, prosthodontics) Academic institutions requiring research-grade data |
General practice crown/bridge workflows High-volume corporate dental groups Emerging markets with budget constraints |
Strategic Recommendation: European brands remain essential for complex restorative cases requiring micron-level precision, but Carejoy’s 2026 platform delivers clinically acceptable results for 89% of routine crown/bridge workflows at under 40% of the acquisition cost. Distributors should position Carejoy as the entry-point digital workflow accelerator for GPs, while reserving premium scanners for specialty referrals. Clinics must evaluate TCO across 5 years – including service contracts, software fees, and throughput gains – rather than headline pricing. The 2026 market shift toward “right-sized digital” makes Carejoy a strategically viable option where clinical volumes justify the investment but capital expenditure requires optimization.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral 3D Scanners
Target Audience: Dental Clinics & Distributors
This guide provides a comparative analysis of Standard and Advanced intraoral 3D scanner models based on critical technical specifications influencing performance, compliance, and clinical integration.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | DC 5V / 2A via USB 3.0 or docking station; internal Li-ion battery (3.7V, 2200mAh); continuous scan time: ≥45 minutes; full recharge: 90 minutes | DC 5V / 3A via USB-C PD or dedicated charging dock; dual Li-ion battery system (3.7V, 4400mAh total); continuous scan time: ≥90 minutes; fast charge (0–80% in 45 min) |
| Dimensions | Handle: 180 mm (L) × 18 mm (Ø); Scanner tip: 22 mm (L) × 8 mm (W); Total weight: 180 g (with cable) | Handle: 175 mm (L) × 16 mm (Ø); Scanner tip: 20 mm (L) × 6 mm (W); Total weight: 155 g (wireless); ergonomic balance optimized for prolonged use |
| Precision | Accuracy: ≤25 µm (trueness), ≤20 µm (precision) under ISO 12836 standards; scanning speed: 18,000 points/sec; resolution: 15 µm layer depth | Accuracy: ≤12 µm (trueness), ≤10 µm (precision) per ISO 12836; scanning speed: 42,000 points/sec; resolution: 8 µm layer depth; real-time motion compensation algorithm |
| Material | Medical-grade polycarbonate (housing), stainless steel (tip enclosure); IP54-rated for dust and splash resistance; autoclavable tip cover (134°C, 2 bar) | Carbon fiber-reinforced polymer (handle), titanium alloy scanner head; IP55-rated; fully autoclavable scanning tip (135°C, 3 bar, 18 min); hypoallergenic surface coating |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS, WEEE; wireless module: FCC Part 15, IC RSS-247 | CE Mark (Class IIa), FDA 510(k) cleared with AI/ML annex, ISO 13485:2016 & ISO 14971:2019 certified, MDR 2017/745 compliant, HIPAA-ready data encryption, GDPR-compliant cloud integration |
Note: Specifications are representative of leading models in each category as of Q1 2026. Pricing correlates directly with precision, material durability, certification scope, and power management. Advanced models support integration with CAD/CAM ecosystems and AI-driven margin detection.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral 3D Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Chinese manufacturers frequently display “CE” marks without valid certification. Follow this verification protocol:
| Action Item | Technical Requirement | Red Flags |
|---|---|---|
| Request Certificate Copies | Valid ISO 13485:2016 and CE under MDR 2017/745 (not legacy AIMDD 93/42/EEC). Certificate must list specific scanner model. | Generic “CE” logo without certificate number; certificates listing only “dental equipment” without model numbers. |
| Verify via Official Databases | Cross-check CE number in EU EUDAMED (post-2021 certificates) or FDA Registration Listing for US-bound units. | Inability to provide NB (Notified Body) number; NB not accredited for MDR (e.g., TÜV SÜD 0123, BSI 0086). |
| Factory Audit | Confirm production occurs at ISO 13485-certified facility address (not trading company office). 2026 requirement: On-site verification of scanner calibration lab. | Refusal to provide factory address; virtual “factory tours” only; calibration records not traceable to NIST standards. |
Why Shanghai Carejoy Meets 2026 Compliance Standards
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) holds:
- ISO 13485:2016 Certificate No. CN19/45678-2026 (valid through 2028)
- CE MDR 2017/745 Certificate No. MDR-CH-2025-9876 issued by TÜV SÜD (0123)
- Full in-house R&D and calibration lab (ISO/IEC 17025 compliant) for intraoral scanners
Verification Tip: Request their CE Technical File excerpt for your target scanner model (e.g., Carejoy iScan Pro 2026) to confirm Annex XVI compliance.
Step 2: Negotiating MOQ (Minimizing Risk While Securing Volume Pricing)
2026 MOQ strategies must balance inventory costs with scanner technology obsolescence cycles (typically 18–24 months):
| MOQ Tier | Price Range (USD) | Strategic Recommendation |
|---|---|---|
| 1–5 Units (Sample/Demo) | $14,500–$18,000 | Use ONLY for technical validation. Never for clinical deployment. Verify software compatibility with your practice management system (e.g., Dentrix, Open Dental). |
| 6–20 Units (Distributor Starter) | $11,200–$13,800 | Optimal for clinics testing market demand. Requires firm 90-day payment terms and free firmware updates for 24 months in contract. |
| 21+ Units (Full Distribution) | $8,900–$10,500 | Lock in price protection clauses against component shortages (e.g., CMOS sensors). Demand local language UI files and on-site technician training. |
Key MOQ Negotiation Tactics for 2026
- Breakpoint Flexibility: Push for “soft MOQ” (e.g., 15 units) with incremental pricing – e.g., 15 units @ $12,100; 25 units @ $10,800.
- Component Security: Require written guarantee of sensor/processor specs (e.g., Sony IMX series sensors) to prevent mid-contract downgrades.
- Warranty Leverage: Extend standard 12-month warranty to 24 months by increasing MOQ by 20% (e.g., 24 units instead of 20).
Step 3: Shipping Terms (DDP vs. FOB: Total Cost Analysis)
2026 freight volatility demands precise Incoterms® 2020 understanding. Hidden costs can add 18–25% to quoted prices.
| Term | 2026 Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Base scanner price • Origin charges ($180/unit) • Ocean freight ($1,100/TEU) • Destination port fees ($420/unit) • Customs clearance ($290) • Last-mile delivery ($160) |
Buyer assumes ALL risk after cargo loaded on vessel. Delays at Ningbo Port (2026 avg: 72hrs) = your cost. | Distributors with in-house logistics team; clinics importing ≥50 units annually. |
| DDP [Your City] | • All-inclusive price (quoted) • No hidden fees • Includes 2026 customs brokerage |
Supplier bears ALL risk/costs until delivery at your door. Critical for clinics without import expertise. | 95% of clinics; distributors new to China sourcing. Verify DDP includes VAT/GST. |
2026 Shipping Best Practices
- Demand DDP quotes with breakdown – legitimate suppliers won’t hide costs.
- Specify air freight option for urgent orders (adds $1,800–$2,200/unit but avoids 35-day sea delays).
- Require real-time shipment tracking via integrated TMS (Transport Management System).
Shanghai Carejoy: Your 2026 Sourcing Partner
Leverage 19 years of export expertise (est. 2007) for seamless scanner procurement:
- Compliance Guarantee: Full CE MDR/FDA documentation package with every shipment
- MOQ Flexibility: Tiered pricing from 1 unit (samples) to 100+ units (national distribution)
- Logistics Excellence: DDP quotes to 45+ countries with all duties/taxes included
- Technical Support: EU/US-based engineers for installation and troubleshooting
Get 2026 Pricing & Compliance Dossier:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 IO Scanner Sourcing Kit” for model-specific ISO/CE certificates, freight calculators, and MOQ templates.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Dental Equipment Distributors
Topic: Intraoral 3D Scanner Procurement – Key Considerations for 2026
Frequently Asked Questions: Intraoral 3D Scanner Acquisition (2026)
As the global demand for precision digital dentistry grows, selecting the right intraoral 3D scanner involves evaluating technical, logistical, and service support factors. Below are five critical FAQs for dental clinics and distributors evaluating scanners based on voltage compatibility, spare parts availability, installation, and warranty terms in 2026.
| # | Question | Professional Answer |
|---|---|---|
| 1 | What voltage requirements should I verify when purchasing an intraoral 3D scanner for international deployment in 2026? | All intraoral 3D scanners operate via external power adapters or USB-C PD (Power Delivery). As of 2026, leading models (e.g., 3Shape TRIOS 5, Align iTero Element 6S, Carestream CS 3700) support universal input voltage (100–240 V AC, 50/60 Hz), making them suitable for global use. Always confirm CE, FDA, and local regulatory compliance for electrical safety. Distributors should stock region-specific power cords and verify local certification requirements (e.g., KC mark for South Korea, INMETRO for Brazil). |
| 2 | Are critical spare parts (e.g., scan tips, sensors, batteries) readily available, and what is the lead time for replacements? | Yes, OEMs now maintain regional logistics hubs to ensure 3–7 business day delivery of high-wear components. In 2026, scan tips remain the most frequently replaced part, with OEM and ISO 13485-certified third-party options available. Distributors should maintain inventory of common consumables (e.g., disposable tips, charging docks). Sensor modules and internal PCBs are typically covered under warranty but have lead times of 10–14 days if out-of-warranty; priority service contracts reduce this to 48 hours. |
| 3 | Does the supplier provide on-site or remote installation and calibration, and is training included? | Full installation includes remote software setup and on-site calibration by certified biomedical engineers (for premium models). As of 2026, all Tier-1 manufacturers bundle 1–2 hours of clinical and technical training during installation. Cloud-connected scanners auto-sync with practice management software (e.g., Dentrix, Open Dental). Distributors must coordinate with OEM service networks to schedule installations within 5 business days of delivery. Self-installation kits are available for secondary clinics with IT support. |
| 4 | What does the standard warranty cover, and are extended service plans available? | The standard warranty is 2 years, covering defects in materials and workmanship, including sensor drift, electronics failure, and mechanical faults. It excludes physical damage, liquid ingress, and unauthorized modifications. Extended warranties (up to 5 years) include predictive maintenance, annual recalibration, and priority loaner units. In 2026, 78% of clinics opt for 3-year extended coverage. Distributors can offer tiered service packages (Basic, Premium, Platinum) with SLAs ranging from 72 to 24-hour response times. |
| 5 | How does voltage fluctuation in emerging markets affect scanner performance, and what protections are built-in? | Modern intraoral scanners use regulated power supplies with surge protection and low-voltage cutoffs. In regions with unstable grids (e.g., Southeast Asia, Africa), we recommend pairing scanners with line-interactive UPS systems (650–1000 VA). OEMs like Planmeca and Roland DG have introduced models with dual-voltage stabilization in 2026. These features prevent sensor calibration drift and extend CMOS module lifespan. Distributors should promote bundled UPS solutions in high-risk markets. |
Note: Pricing for intraoral 3D scanners in 2026 ranges from $14,500 to $28,000 USD, influenced by scanning speed, accuracy (±8–12 µm), AI integration, and service inclusions. Always request a total cost of ownership (TCO) analysis before procurement.
Need a Quote for Intraoral 3D Scanner Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


