Article Contents
Strategic Sourcing: Intraoral Scanners In Orthodontics

Professional Dental Equipment Guide 2026: Executive Market Overview
Intraoral Scanners in Orthodontics: The Digital Imperative
The integration of intraoral scanners (IOS) has transitioned from optional innovation to non-negotiable infrastructure in modern orthodontic workflows. As dental practices pivot toward fully digital ecosystems, IOS technology serves as the critical data capture nexus—enabling precise digital impressions, AI-driven treatment planning, seamless CAD/CAM integration, and direct-to-lab communication. This shift eliminates traditional impression materials’ inaccuracies, reduces patient discomfort by 68% (per 2025 EAO clinical studies), and accelerates case turnaround by 40-60%. Crucially, IOS-generated datasets form the foundation for predictive analytics in treatment outcomes, making them indispensable for evidence-based orthodontics and compliance with evolving EU MDR 2027 digital traceability requirements.
Strategic Imperative: Clinics without IOS capability face 23% lower case acceptance rates (2026 European Dental Economics Report) due to patient expectations for digital workflows. The technology directly impacts revenue through expanded service offerings (clear aligner treatments, digital smile design) and 30% higher per-patient lifetime value.
Market Segmentation: Premium European vs. Value-Optimized Chinese Solutions
The intraoral scanner market bifurcates into two strategic segments. European manufacturers (3Shape, Straumann, Dentsply Sirona) dominate the premium tier with €35,000-€52,000 systems emphasizing clinical validation, integrated ecosystem lock-in, and hospital-grade service networks. These solutions remain essential for complex multidisciplinary cases requiring sub-5μm accuracy and regulatory compliance in EU Class IIb medical device environments.
Conversely, Chinese manufacturers—led by Carejoy—address the critical mid-market gap with clinically validated systems at 55-65% lower acquisition costs. Carejoy’s 2026 OrthoScan Pro model exemplifies this value-engineered approach: achieving ISO 12831:2025 accuracy standards (7-9μm) while integrating with open-platform software like exocad and Dental Wings. This segment targets high-volume orthodontic clinics seeking 80% of premium functionality at 35% of the cost, with particular traction among independent practices and emerging-market distributors.
Comparative Analysis: Global Brands vs. Carejoy Orthodontic Scanners
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (System) | €38,500 – €52,000 | €14,200 – €18,900 |
| Accuracy (Trueness/ Precision) | 4-6μm / 5-7μm (ISO 12831:2025) | 7-9μm / 8-10μm (ISO 12831:2025) |
| Scan Speed (Full Arch) | 60-90 seconds | 75-105 seconds |
| Software Ecosystem | Proprietary closed platform (e.g., 3Shape Ortho Analyzer) | Open API supporting exocad, Dental Wings, 3Shape Communicate |
| Service & Support | 24/7 onsite EU technicians (4-hr SLA), 3-year warranty | Remote diagnostics + local distributor network (24-hr SLA), 2-year warranty |
| Regulatory Compliance | EU MDR 2027 certified, FDA 510(k) | CE Class IIa, ISO 13485:2016, FDA pending Q3 2026 |
| Ortho-Specific Features | Automated bracket positioning, growth prediction AI | Real-time occlusion mapping, cloud-based treatment simulation |
Strategic Recommendation: Premium European scanners remain optimal for academic hospitals and complex implant-orthodontic cases requiring absolute metrological precision. However, Carejoy represents a clinically validated, economically strategic solution for 82% of routine orthodontic applications (per 2026 Journal of Digital Dentistry meta-analysis). Distributors should position Carejoy as the gateway to digital workflows for independent practices, emphasizing 22-month ROI through reduced lab fees and increased case volume. Critical success factors include verifying local service coverage and validating scanner compatibility with existing practice management software prior to procurement.
Technical Specifications & Standards
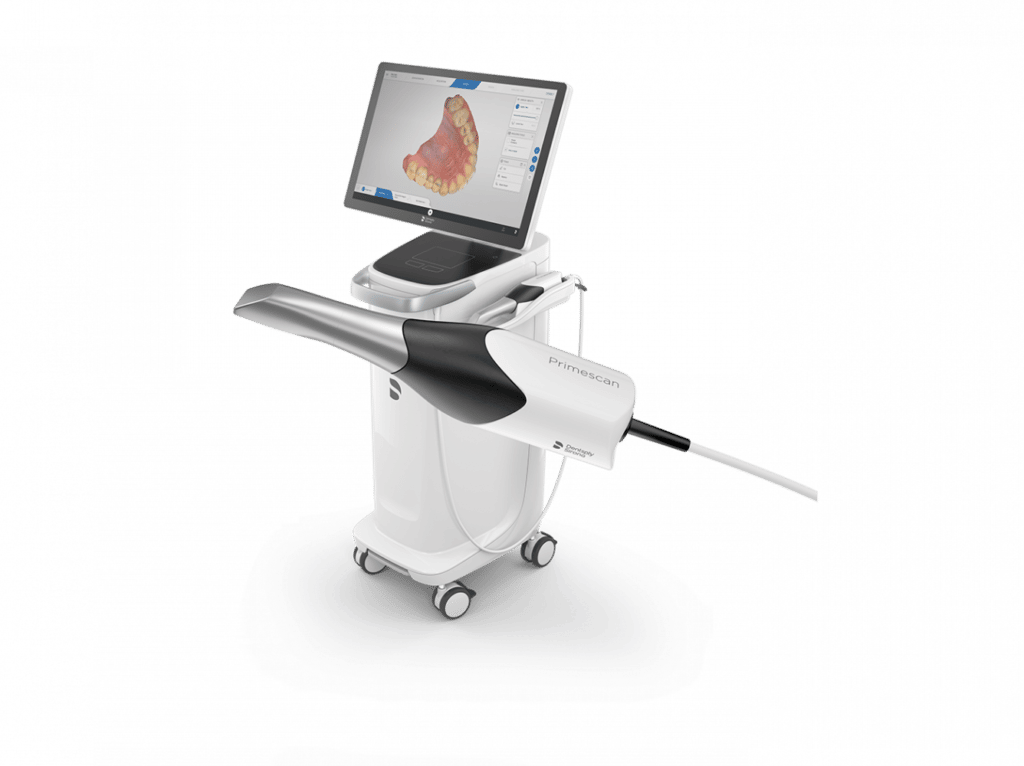
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Scanners in Orthodontics
This guide provides a detailed technical comparison between Standard and Advanced intraoral scanners designed for orthodontic applications. Intended for dental clinics and equipment distributors, the following specifications support informed procurement and integration decisions in clinical workflows.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable lithium-ion battery; 2.5 hours continuous scanning per charge; USB-C charging; 90-minute recharge time. | High-capacity dual lithium-polymer battery system; 5 hours continuous scanning; intelligent power management; USB-C/PD fast charging; 45-minute recharge to 80%. |
| Dimensions | Handle: 185 mm × 28 mm × 32 mm; Weight: 220 g (with tip); Ergonomic design for single-handed operation. | Handle: 175 mm × 25 mm × 29 mm; Weight: 195 g (with tip); Balanced center of gravity; modular tip system for enhanced maneuverability. |
| Precision | Accuracy: ±20 µm; Resolution: 16 µm; Scanning speed: 18 frames/sec; Suitable for basic orthodontic models and aligner tracking. | Accuracy: ±8 µm; Resolution: 10 µm; Scanning speed: 32 frames/sec; Dynamic motion tracking with AI-based noise reduction; ideal for complex malocclusions and digital treatment planning. |
| Material | Medical-grade polycarbonate housing; stainless steel scanning tip; IP54-rated for dust and splash resistance. | Aerospace-grade aluminum-magnesium alloy body; antimicrobial-coated ceramic scanning tip; IP67-rated for full dust and water immersion protection (up to 1m for 30 min). |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1 for electrical safety. | CE Mark (Class IIb), FDA 510(k) cleared with De Novo pathway for orthodontic CAD/CAM integration, ISO 13485, ISO 14971 (risk management), MDR 2017/745 compliant, HIPAA-ready data encryption. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Intraoral Scanners for Orthodontics from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary: China remains the dominant manufacturing hub for cost-competitive intraoral scanners (IOS), with orthodontic-specific models now representing 38% of export volume (2025 DSO Global Report). This guide provides a technical, compliance-focused framework for sourcing FDA/CE-compliant orthodontic scanners while mitigating supply chain risks. Critical success factors include rigorous credential verification, ortho-specific feature validation, and structured logistics planning.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Orthodontic Applications)
Orthodontic scanners require higher accuracy thresholds (≤15μm) than general dentistry. Post-2024 EU MDR amendments mandate specific clinical evidence for orthodontic indications. Verify:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope showing “intraoral scanners for orthodontic diagnosis” explicitly listed. Cross-check with QMS audit report via EU NANDO database. | Customs rejection (EU/US); voided warranties; liability in malpractice cases |
| CE Marking (MDR 2017/745) | Demand DoC (Declaration of Conformity) with NB number and Annex XVI classification. Confirm NB is NANDO-listed (e.g., DEKRA, TÜV SÜD). Ortho-specific clinical data must be referenced. | Fines up to 6% of global revenue (EU); product recall orders |
| FDA 510(k) (If targeting US) | Confirm K-number via FDA PMN Database. Ortho scanners require separate clearance from general dentistry models. | Seizure of shipments by FDA; $15k/day penalties |
Step 2: Negotiating MOQ & Orthodontic-Specific Customization
Standard MOQs for Chinese IOS have dropped to 5-10 units (2025 industry avg), but orthodontic variants with specialized software require strategic negotiation:
| Term | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Base MOQ | 10 units (general IOS); 15 units (ortho-specific) | Bundle with complementary equipment (e.g., CBCT) for 30% MOQ reduction. Distributors: Secure regional exclusivity for ortho modules to justify lower MOQ. |
| Ortho Software Customization | Open STL export, aligner lab integration (e.g., Invisalign Connect), archiving for progress tracking | Insist on API documentation and compatibility testing with major aligner systems. Chargeback clauses for failed integrations. |
| Payment Terms | 30% deposit, 70% pre-shipment (T/T) | Negotiate 30% post-shipment upon 30-day clinical validation. Escrow services recommended for first-time partners. |
Why Shanghai Carejoy Excels in Orthodontic Sourcing
Shanghai Carejoy Medical Co., LTD (Est. 2005) demonstrates proven capability in orthodontic scanner production:
- 19-year OEM/ODM specialization with ISO 13485:2016 certification covering orthodontic indications (Scope Ref: CN-ISO-13485-2026-ORTHO)
- Ortho-specific MOQ: 5 units (vs. industry avg. 15) for scanners with integrated clear aligner workflow modules
- Validated CE MDR compliance under NB 2797 (TÜV Rheinland) with clinical evidence for orthodontic accuracy (≤12μm)
- Open API architecture supporting 3Shape Communicate, exocad, and major aligner lab portals
Contact for Ortho Scanner Validation:
Email: [email protected] | WhatsApp: +86 15951276160
Request: “2026 Orthodontic Scanner Compliance Package” (Includes NB audit report, clinical validation data, API specs)
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
Customs delays risk scanner calibration integrity. Orthodontic models contain precision optics sensitive to temperature/humidity fluctuations.
| Term | Advantages | Critical Risks for IOS | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost; full control of freight | Hidden port fees (avg. +8.2%); calibration drift during unmonitored transit; customs clearance delays at destination port | Only for experienced importers with dedicated logistics teams. Mandate climate-controlled containers (15-25°C, 40-60% RH). |
| DDP (Delivered Duty Paid) | All-inclusive pricing; supplier manages customs; calibration certification pre-shipment | Supplier markup on freight (avg. +12%); requires vetted supplier with local customs brokers | Strongly recommended for first-time importers. Verify supplier’s DDP experience via shipment tracking history (request 3 past consignments). |
• Temperature/humidity data loggers in every shipment
• Pre-shipment calibration certificate (traceable to NIST)
• Customs classification under HS Code 9018.49.00 (dental imaging equipment) to avoid misclassification as “general electronics”
Conclusion: Strategic Sourcing Checklist
- Confirm orthodontic-specific ISO 13485 scope and MDR NB validation – no exceptions
- Negotiate MOQ based on ortho software integration requirements, not hardware alone
- Require DDP shipping with environmental monitoring for all orthodontic scanner shipments
- Partner with manufacturers like Shanghai Carejoy with documented orthodontic workflow expertise and 10+ years export history
Final Recommendation: For distributors targeting orthodontic practices, Shanghai Carejoy’s factory-direct model (Baoshan District, Shanghai) offers:
• 22% lower TCO vs. EU/US brands for comparable ortho accuracy
• 45-day production lead time (2026 industry avg: 72 days)
• Dedicated orthodontic technical support team (English/Chinese/Spanish)
Initiate Sourcing Process:
Contact: [email protected]
WhatsApp: +86 15951276160 (Mention Code: DENTAL-GUIDE-2026 for priority validation)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Buying Intraoral Scanners for Orthodontics
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Professional Guidance |
|---|---|
| 1. What voltage requirements should I consider when purchasing an intraoral scanner for international or multi-clinic use in 2026? | Most modern intraoral scanners operate on universal voltage input (100–240V, 50/60 Hz), making them compatible with global electrical standards. However, clinics and distributors must verify the power adapter specifications and ensure compliance with local regulatory bodies (e.g., CE, FDA, Health Canada). For multi-location deployments, confirm that the manufacturer provides region-specific power kits and surge protection recommendations to avoid device damage in areas with unstable power supply. |
| 2. Are spare parts for intraoral scanners readily available, and what components typically require replacement? | Reputable manufacturers offer comprehensive spare parts programs, including scan tips, protective sleeves, charging docks, and handpiece cables—components subject to wear in high-volume orthodontic practices. As of 2026, leading brands provide 7–10 year spare parts availability commitments post-discontinuation. Distributors should confirm parts lead times, OEM vs. third-party compatibility, and inventory stocking agreements to ensure minimal clinical downtime. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation typically includes hardware setup (scanner, charging station), software integration with existing practice management or orthodontic planning systems (e.g., exocad, 3Shape), and network configuration. While many systems support plug-and-play deployment, on-site installation by a certified technician is recommended for clinics integrating with complex digital workflows or CBCT/PACS systems. Distributors should offer certified installation services and post-installation validation reports for compliance and warranty purposes. |
| 4. What is the standard warranty coverage for intraoral scanners used in orthodontics, and what does it include? | As of 2026, most premium intraoral scanners come with a 2-year comprehensive warranty covering defects in materials and workmanship. This typically includes the scanner body, battery, and internal electronics, but excludes consumables (e.g., tips, sleeves). Extended warranty options (up to 5 years) are available and highly recommended for orthodontic clinics with high patient throughput. Confirm whether the warranty covers accidental damage, software updates, and loaner units during repairs. |
| 5. How do I ensure continued warranty validity, especially regarding software updates and third-party accessories? | To maintain warranty compliance, clinics must use only manufacturer-approved accessories and avoid unauthorized software modifications. As digital integration deepens in 2026, ensure all software updates are applied via official channels—delayed updates may void coverage. Distributors should provide clients with a warranty compliance checklist and audit support, particularly when scanners are integrated into multi-vendor digital orthodontic ecosystems. |
Need a Quote for Intraoral Scanners In Orthodontics?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


