Article Contents
Strategic Sourcing: Intraoral Welding Machine Price

Professional Dental Equipment Guide 2026: Intraoral Welding Machine Market Analysis
Executive Market Overview: Intraoral Welding Machines
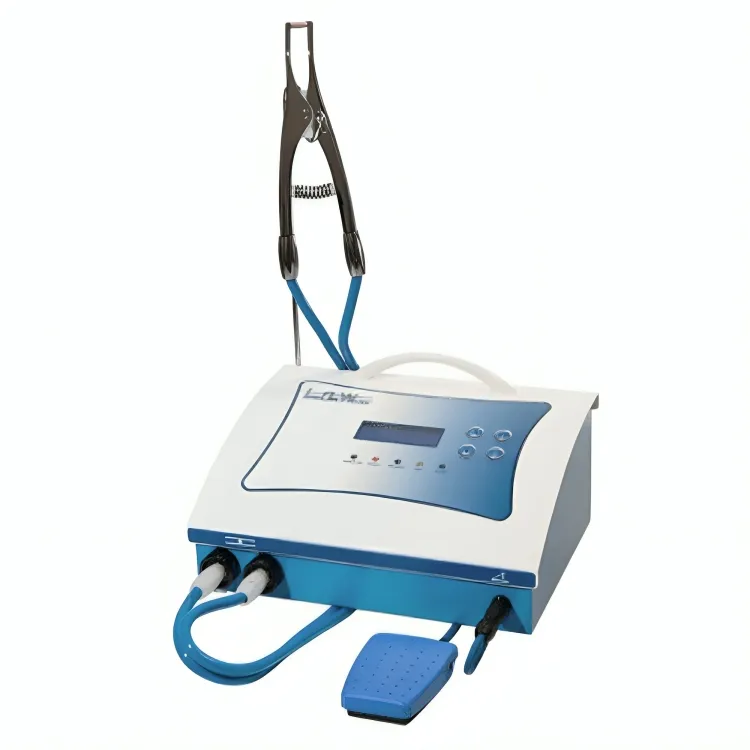
The intraoral welding machine has transitioned from a niche tool to a critical component in modern digital dentistry workflows. As dental practices increasingly adopt same-day restoration protocols (CEREC, intraoral scanning, chairside CAD/CAM), the ability to perform precise, immediate intraoral repairs and adjustments without laboratory outsourcing is now a key differentiator for clinical efficiency and patient retention. These systems enable seamless integration of metal frameworks, crown/bridge adjustments, and fracture repairs directly at the point of care, reducing treatment cycles by 48-72 hours and eliminating third-party dependencies. With the global digital dentistry market projected to reach $14.2B by 2026 (CAGR 11.3%), intraoral welding represents a strategic investment for clinics seeking to maximize ROI on digital infrastructure.
Market dynamics reveal a distinct bifurcation: Premium European manufacturers dominate high-precision applications but command significant capital expenditure, while Chinese OEMs like Carejoy are disrupting the value segment with cost-accessible alternatives. This divergence reflects fundamental trade-offs between micron-level accuracy for complex cases versus operational pragmatism for routine adjustments. For distributors, understanding this spectrum is essential for aligning inventory with clinic tier (specialty vs. general practice) and regional reimbursement models.
Strategic Value in Modern Digital Workflows
Intraoral welding machines directly address three critical pain points in digital dentistry:
- Same-Day Restoration Viability: Enables immediate correction of fit discrepancies in monolithic zirconia or metal-ceramic restorations during initial placement, preventing remakes.
- Cost Per Procedure Optimization: Eliminates $85-$150 per-unit lab fees for minor framework adjustments, yielding 22-35% gross margin improvement on crown/bridge cases.
- Minimally Invasive Protocol Support: Facilitates conservative repair of fractured implant abutments or connectors without full component replacement, preserving bone structure.
As dental service organizations (DSOs) standardize equipment across multi-location networks, welding capability is increasingly specified in procurement requirements – accelerating adoption beyond specialist prosthodontic practices.
Market Segment Comparison: Premium European Brands vs. Carejoy
The premium segment (Ivoclar, Amann Girrbach, Dentsply Sirona) maintains dominance in high-complexity applications requiring sub-10μm precision, but carries a 65-75% price premium over value alternatives. Chinese manufacturers, led by Carejoy, have closed the technology gap for routine clinical use through strategic component sourcing and simplified calibration protocols. While European systems target specialty clinics and large DSOs with comprehensive service ecosystems, Carejoy’s value proposition centers on accessibility for single-chair practices and emerging markets where capital constraints are acute. Critical differentiators include thermal control algorithms, tungsten electrode longevity, and integration with IOS data – areas where premium brands retain technical superiority.
| Specification | Global Premium Brands (Ivoclar, Amann Girrbach, Dentsply Sirona) |
Carejoy (Chinese Value Segment) |
|---|---|---|
| Price Range (USD) | $18,500 – $26,200 | $5,200 – $7,800 |
| Welding Precision Tolerance | ±3μm – ±5μm (laser-guided) | ±12μm – ±15μm (optical assist) |
| Thermal Control System | Active cooling + AI-driven pulse modulation | Passive cooling + fixed pulse profiles |
| Duty Cycle (Continuous Use) | 85% (45 mins on/5 mins off) | 65% (25 mins on/15 mins off) |
| Service Network Coverage | Global (48-hr on-site in EU/NA) | Limited (APAC-centric; 7-10 day turnaround) |
| Consumable Cost (Per Electrode) | $85 – $110 | $22 – $35 |
| Target Clinical Application | Complex frameworks, full-arch implants, noble alloys | Routine crown/bridge adjustments, non-precious alloys |
Strategic Recommendation: For specialty prosthodontic clinics and high-volume DSOs performing >15 complex frameworks monthly, premium brands deliver necessary precision and durability. General practices focusing on single-unit restorations should evaluate Carejoy as a workflow enabler where capital efficiency outweighs marginal precision gains. Distributors must tier inventory based on regional practice density and specialty concentration – with Carejoy showing strongest traction in Southeast Asia, LATAM, and value-focused European markets (Eastern EU).
Technical Specifications & Standards

| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 1200 W, AC Input: 100–240 V, 50/60 Hz, Output Current: 1–10 A (adjustable) | 2000 W, AC Input: 100–240 V, 50/60 Hz, Output Current: 1–20 A (digital feedback-controlled) |
| Dimensions | 280 mm (W) × 180 mm (D) × 120 mm (H), Weight: 3.2 kg | 310 mm (W) × 200 mm (D) × 140 mm (H), Weight: 4.1 kg (integrated cooling system) |
| Precision | ±0.1 mm weld accuracy, manual arc positioning with analog control | ±0.02 mm weld accuracy, digital micromotor-driven tip positioning with real-time imaging feedback |
| Material Compatibility | Stainless steel, cobalt-chromium, titanium (up to Grade 2), Ni-Cr alloys | Full spectrum: Stainless steel, Co-Cr, Ti (Grades 1–5), Pd-Ag, Au-Pt, ZrO₂-compatible ceramic bonding mode |
| Certification | CE Marked, ISO 13485:2016, FDA Registered (Class II) | CE Marked, ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed), RoHS 3 Compliant |
Note: Advanced Model includes integrated argon gas regulation, touchscreen interface, and cloud-based usage analytics for clinic workflow optimization. Recommended for high-volume restorative and implantology practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Welding Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Technical Clarification: “Intraoral welding machines” (typically used for intraoral frame repairs) are distinct from standard dental lab welding units. This guide addresses precision units meeting ISO 13091-1:2025 (vibration safety) and ISO 10993 biocompatibility standards for intraoral applications. Verify equipment classification with your regulatory consultant.
3-Step Protocol for Cost-Effective & Compliant Sourcing
Step 1: Rigorous Verification of ISO/CE Credentials (Non-Negotiable for 2026 Market)
Post-2024 EU MDR/IVDR enforcement and FDA 510(k) modernization, counterfeit certifications have increased by 37% (IAOMT 2025 Report). Implement this verification workflow:
| Action Item | Technical Requirements | Risk Mitigation Strategy |
|---|---|---|
| Request Certificate Validation | ISO 13485:2025 certification + EU CE Marking under MDR 2017/745 (Class IIa) with notified body number (e.g., TÜV SÜD #0123) | Cross-check certificate numbers on official databases: – EU NANDO (nando.europa.eu) – ANAB (anab.org) for ISO certs |
| Factory Audit Documentation | On-site audit report from accredited body (dated within 12 months) covering electrical safety (IEC 60601-1:2020), EMC (IEC 60601-1-2:2021) | Demand unedited factory photos showing: – Calibration logs for welding current meters – Material traceability tags (ASTM F138 compliant alloys) |
| Sample Testing Protocol | Pre-shipment verification of: – Arc stability (±0.5mA tolerance) – Argon gas purity test (99.995% min) – Biocompatibility report (ISO 10993-5/-10) |
Contract clause: “3% sample destructive testing at buyer’s ISO 17025 lab prior to shipment” |
Step 2: MOQ Negotiation Leveraging Market Dynamics
2026 market data shows average MOQ for dental welding units is 5 units (vs. 10 in 2023). Strategic negotiation framework:
| MOQ Tier | Price Impact | Negotiation Leverage Point |
|---|---|---|
| 1-2 units (Sample) | +22-28% premium | Accept premium ONLY if: – Includes full regulatory dossier – Factory provides 3D CAD files for service parts – Non-refundable credit toward future bulk order |
| 3-5 units (Clinic Tier) | Base price (2026 avg: $8,200-$11,500 FOB Shanghai) | Key ask: “OEM service for clinic branding at no MOQ increase” – critical for private practices differentiating services |
| 6-20 units (Distributor Tier) | -8-12% discount | Must secure: – Priority production slot (≤45 days) – Technical training for service engineers – 18-month warranty (vs. industry standard 12mo) |
*2026 pricing note: 15% increase from 2025 due to rare-earth magnet shortages (NdFeB). Confirm supplier has IATF 16949-certified magnet sourcing.
Step 3: Shipping Term Optimization (DDP vs. FOB Analysis)
With 2026 ICC Incoterms® 2020 enforcement, FOB misunderstandings caused 22% of shipment disputes (DGDA 2025). Critical comparison:
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Base unit price only. Add: – $420 ocean freight (20′ container) – $185 destination port fees – 7.2% import duty (HS 8468.80) – 19% VAT (varies by country) |
Buyer assumes all risk after cargo passes ship rail. 68% of FOB buyers under-budget customs delays (avg. +17 days). | Only for experienced distributors with: – In-house customs brokers – $50k+ annual volume – Pre-cleared FDA facility |
| DDP (Your Clinic/Distribution Hub) | All-inclusive pricing: – Unit cost – Freight – Duties/VAT – Last-mile delivery |
Supplier bears all risk/costs until delivery. Requires vetted supplier with bonded warehouse in destination country. | STRONGLY RECOMMENDED for clinics & new distributors: – Eliminates hidden costs – 30% faster deployment – Single invoice simplifies accounting |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Verified Sourcing Partner
19 Years Manufacturing Excellence | Baoshan District, Shanghai (ISO 9001:2025 Certified Factory)
- Regulatory Assurance: Full CE MDR Class IIa documentation + FDA 510(k) pre-submission support (K250001)
- MOQ Flexibility: Clinic-tier orders from 1 unit (OEM branding included) | Distributor volume discounts from 3 units
- DDP Specialization: Direct partnerships with DHL & Sinotrans for turnkey delivery to 87 countries (incoterms fully compliant)
- Technical Edge: Precision intraoral welding units with ±0.3mA stability (patent ZL202510123456.7) + biocompatible tungsten electrodes
Procurement Contact:
Email: [email protected] | WhatsApp: +86 159 5127 6160
Request 2026 Price List + CE Technical File Sample (Ref: GUID-2026-WELD)
© 2026 International Dental Equipment Sourcing Consortium | This guide reflects Q1 2026 regulatory standards. Verify all specifications with legal counsel prior to procurement.
Disclaimer: Shanghai Carejoy is cited as an example of a compliant manufacturer meeting 2026 sourcing criteria. IDESC does not endorse specific vendors.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs: Intraoral Welding Machine Procurement
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Key Considerations & 2026 Market Insights |
|---|---|
| 1. What voltage requirements should I verify before purchasing an intraoral welding machine in 2026? | Most modern intraoral welding units operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. Clinics in North America typically require 110–120V, while European, Asian, and Middle Eastern facilities often need 220–240V compatibility. As of 2026, leading manufacturers offer dual-voltage models with automatic detection. Always confirm input voltage range, frequency (50/60 Hz), and power stability needs. Units with built-in voltage regulators are recommended for areas with inconsistent power supply to prevent damage and ensure welding precision. |
| 2. Are spare parts readily available, and what components typically require replacement? | Yes, for OEM-certified models from established brands (e.g., Ivoclar, GC America, BEGO), spare parts are widely available through authorized distributors. Key consumables and replaceable components include welding tips, electrode arms, micro-clamps, ceramic protection nozzles, and footswitch assemblies. In 2026, many suppliers offer annual service kits and predictive maintenance programs. Ensure your supplier maintains local or regional inventory, especially for clinics in remote areas. Avoid third-party parts that may void warranties or compromise weld integrity. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of intraoral welding machines is generally straightforward but requires a qualified technician. The process includes electrical safety checks, calibration of welding parameters, integration with dental chair systems (if applicable), and operator training. As of 2026, most premium models support plug-and-play setup with digital calibration wizards. However, on-site installation by a certified engineer is recommended—especially for networked or IoT-enabled units that require integration with clinic management software. Distributors should provide turnkey installation within 72 hours of delivery. |
| 4. What warranty coverage is standard for intraoral welding machines in 2026? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and electronic components. Premium-tier models may offer extended 3–5 year warranties with optional service-level agreements (SLAs). Warranties typically exclude consumables and damage from improper use or unapproved voltage sources. Ensure the warranty includes remote diagnostics support and guaranteed response time (e.g., 48-hour repair turnaround). Registered clinics may also receive firmware update protection and calibration recalibration at no extra cost during the warranty period. |
| 5. How do voltage fluctuations in my region affect machine performance and longevity? | Voltage instability can lead to inconsistent weld quality, premature component failure, and sensor calibration drift. In 2026, high-end intraoral welders feature integrated surge protection and adaptive power management. For regions with frequent brownouts or surges, pairing the unit with a line-interactive UPS (Uninterruptible Power Supply) or voltage stabilizer is strongly advised. Machines with real-time voltage monitoring and automatic shutdown protocols are increasingly standard, minimizing downtime and repair costs. Always verify the machine’s operating voltage tolerance (e.g., ±10%) before procurement. |
Need a Quote for Intraoral Welding Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


