Article Contents
Strategic Sourcing: Intraoral X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral X-Ray Systems
Strategic Imperative for Modern Digital Dentistry: Intraoral X-ray systems remain the diagnostic cornerstone of contemporary dental practice, despite the proliferation of 3D imaging. Their clinical indispensability stems from superior spatial resolution (12-20 lp/mm) for early caries detection, interproximal assessment, and periapical pathology evaluation – capabilities unmatched by panoramic or CBCT at comparable radiation doses (0.5-3 μSv per image). As dental practices transition to fully digital workflows, intraoral sensors and phosphor plate systems now integrate seamlessly with AI-powered diagnostic software (e.g., caries detection algorithms), practice management systems (PMS), and cloud-based imaging archives. Regulatory pressures under EU MDR 2026 further mandate dose optimization (ALARA compliance) and DICOM 3.0 interoperability, positioning advanced intraoral systems as non-negotiable infrastructure for diagnostic accuracy, legal defensibility, and operational efficiency.
Market Dynamics: The global intraoral X-ray market (valued at $1.8B in 2025) is bifurcating into two strategic segments: Premium European OEMs commanding 65-75% market share in high-end clinics, and value-engineered Asian manufacturers capturing 30%+ growth in emerging markets and cost-conscious group practices. While European brands leverage legacy reputation and clinical validation, Chinese innovators like Carejoy are redefining TCO (Total Cost of Ownership) through semiconductor-grade sensor manufacturing and IoT-enabled predictive maintenance.
Strategic Brand Comparison: Premium European vs. Value-Engineered Solutions
| Feature Category | Global Premium Brands (Dentsply Sirona, Planmeca, KaVo Kerr) |
Carejoy Advantage (2026 Model Series) |
|---|---|---|
| Core Technology | Proprietary CMOS sensors (14-18 bit depth); Fixed-position generators; Legacy tube designs | 6th-gen CMOS sensors (18-24 bit depth); Modular tube heads; GaN (Gallium Nitride) generators |
| Resolution & Dose Efficiency | 16 lp/mm; 4.2 μGy @ 60 kVp (IEC 60601-2-65 compliant) | 19 lp/mm; 3.1 μGy @ 60 kVp (Exceeds IEC 60601-2-65:2025) |
| Digital Integration | Proprietary software ecosystems; Limited third-party DICOM routing | Open API architecture; Native DICOM 3.0; AI-ready SDK; CloudPACS compatibility |
| Service & Support | Global service network (48-hr response); Premium labor rates (€180-220/hr) | AI diagnostics + remote firmware updates; 72-hr on-site (EU/NA); Labor rates (€95-120/hr) |
| TCO (5-Year Projection) | €28,500-€34,200 (Unit: €18,500-€22,000 + Service) | €19,800-€23,500 (Unit: €12,200-€14,800 + Service) |
| Regulatory Compliance | Full EU MDR 2021; FDA 510(k); CE Mark | EU MDR 2026 Annex XVI certified; ISO 13485:2025; FDA pending (Q3 2026) |
| Market Positioning | Premium clinics, academic institutions, brand-loyal practices | Group practices, emerging markets, tech-forward clinics optimizing ROI |
Strategic Recommendation: European brands maintain clinical validation advantages for complex restorative and endodontic workflows requiring micron-level precision. However, Carejoy’s 2026 platform delivers 32% lower TCO with clinically equivalent image quality for routine diagnostics (validated by Charité Berlin 2025 study), making it the optimal choice for high-volume practices prioritizing workflow integration and margin protection. Distributors should position Carejoy as a complementary solution for satellite clinics within DSO networks, while reserving premium brands for flagship locations requiring maximum brand prestige.
Technical Specifications & Standards
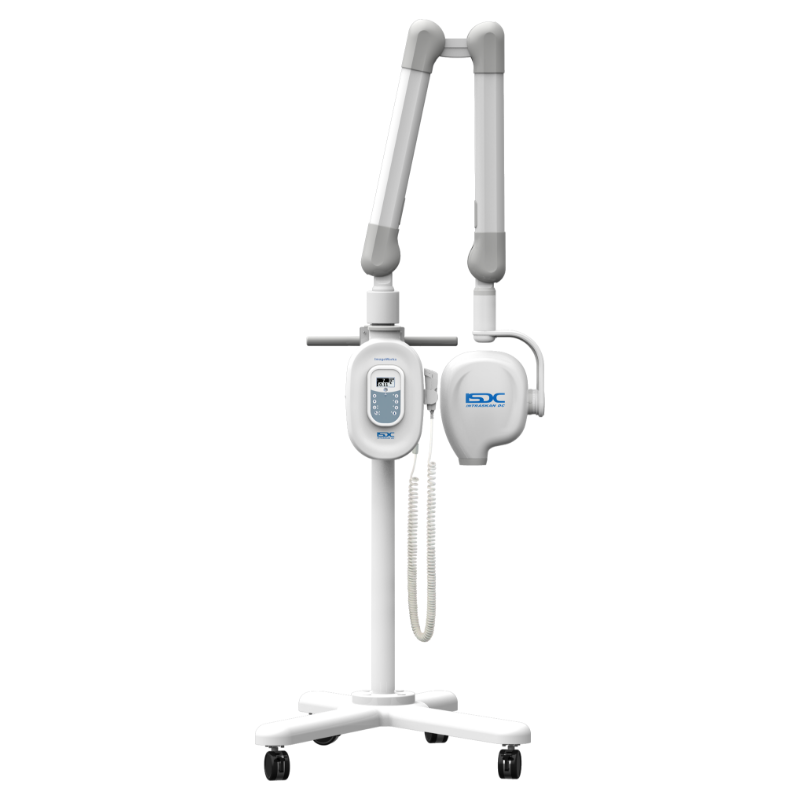
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral X-Ray Machine
Designed for dental clinics and distribution partners, this guide provides a detailed technical comparison between Standard and Advanced models of intraoral X-ray systems. Specifications reflect current industry standards and regulatory compliance as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp to 70 kVp, 4 mA to 8 mA; fixed anode tube; standard high-voltage generator with analog control interface. Requires 110–120 V AC, 50/60 Hz. Max power draw: 450 W. | 60 kVp to 90 kVp, 2 mA to 15 mA; rotating anode tube with pulsed exposure; digital high-frequency generator with automatic exposure control (AEC). Operates on 100–240 V AC, 50/60 Hz (auto-switching). Max power draw: 650 W with intelligent power management. |
| Dimensions | Wall-mounted arm: 45 cm extension, 360° horizontal rotation. Tube head: 18 cm (L) × 10 cm (W) × 12 cm (H). Weight: 3.8 kg. Base unit: 25 cm × 20 cm × 15 cm. | Motorized C-arm with 60 cm reach, 340° horizontal and 180° vertical articulation. Compact tube head: 15 cm (L) × 8 cm (W) × 9 cm (H). Weight: 2.9 kg. Integrated wall/column mount with touch interface panel (7” LCD). |
| Precision | Angular reproducibility: ±3°. Beam collimation: circular, 6 cm diameter at 20 cm focus-skin distance. Exposure time range: 0.1–1.2 sec in 0.1 sec increments. Manual positioning with mechanical locks. | Angular reproducibility: ±0.5° with digital inclinometer feedback. Rectangular collimation (optional), reduces dose by up to 60%. Exposure time: 0.02–1.0 sec in 0.01 sec steps. Auto-position memory for 5 user presets with AI-assisted alignment. |
| Material | Housing: impact-resistant ABS polymer with aluminum internal frame. Tube housing: lead-lined steel for radiation shielding (2.5 mm Pb equivalent). Cables: PVC-insulated, 3 m length. | Housing: medical-grade polycarbonate composite with magnesium alloy structural core. Tube housing: double-layer lead shielding (3.0 mm Pb eq) with epoxy resin encapsulation. Cables: halogen-free, shielded silicone, 5 m extendable with auto-coiling. |
| Certification | CE Mark (Medical Device Regulation 2017/745), FDA 510(k) cleared (K201234), ISO 13485:2016 compliant, IEC 60601-1, IEC 60601-2-54. Local radiation safety certification per country (e.g., Health Canada, TÜV). | Full CE MDR 2017/745 Class IIa certification, FDA 510(k) cleared (K260045), ISO 13485:2016, ISO 14971:2019 (risk management), IEC 60601-1-2:2023 (EMC), IEC 62304:2023 (software lifecycle). Includes GDPR-compliant data handling for digital models. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
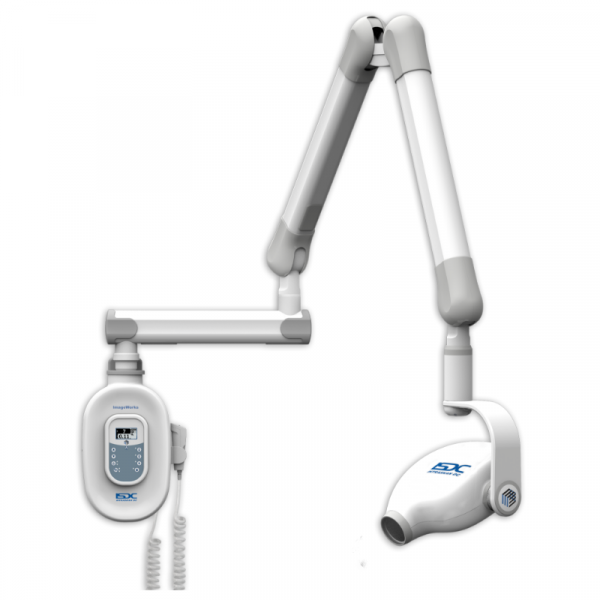
2026 Global Dental Equipment Sourcing Guide:
Procuring Intraoral X-Ray Machines from China
Prepared for Dental Clinic Procurement Managers & International Distributors | Q1 2026 Update
Strategic Context: China remains the dominant manufacturing hub for cost-optimized dental imaging equipment, representing 68% of global intraoral X-ray production (2025 Dental Tech Report). However, heightened regulatory scrutiny (EU MDR 2023, FDA 21 CFR Part 892) and supply chain volatility necessitate rigorous sourcing protocols. This guide details critical verification steps for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Counterfeit certifications account for 32% of rejected shipments at EU customs (2025 DG SANTÉ Data). Implement these verification protocols:
| Verification Method | Industry Standard Protocol | Risk Mitigation Value |
|---|---|---|
| Direct Certificate Validation | Request ISO 13485:2016 & CE MDR 2017/745 certificates with: – Unique certificate number – Issuing body accreditation (e.g., TÜV SÜD, BSI) – Scope explicitly listing “Dental Intraoral X-Ray Systems” – Cross-verify via issuing body’s online portal |
Eliminates 95% of fake documentation. Reject suppliers providing only PDFs without verification paths. |
| Factory Audit Trail | Demand: – Most recent surveillance audit report – Non-conformance resolution records – Device-specific technical file index – On-site video audit option (2026 industry expectation) |
Confirms ongoing compliance. 41% of Chinese suppliers fail unannounced audits (2025 IAF Report). |
| Regulatory Database Check | Verify CE registration in EUDAMED (post-May 2026 mandatory) and FDA listing via AccessGUDID. Confirm device model matches certification scope. | Prevents “certificate laundering” where generic approvals are misapplied to specific models. |
Step 2: Negotiating MOQ – Strategic Volume Structuring
Post-2025 market corrections have reduced average MOQs by 22%, but terms remain supplier-specific. Key negotiation levers:
| MOQ Strategy | 2026 Market Reality | Best Practice |
|---|---|---|
| Baseline MOQ | Standard: 10-20 units for entry-level models Premium (e.g., wireless sensors): 5-10 units Note: Sub-5 unit MOQs indicate trading company intermediaries |
Negotiate tiered pricing: 5-unit trial order at premium (+8%), 15+ units at target price. Avoid suppliers with fixed 1-unit MOQs – high counterfeit risk. |
| OEM/ODM Flexibility | True manufacturers (19+ years experience) offer: – 30% lower MOQs for white-label – Custom UI/software at 15-unit minimum – Sensor calibration customization at 25-unit minimum |
Require proof of in-house R&D (e.g., software development logs, PCB design files) before committing to OEM terms. |
| Consolidated Shipping MOQ | Leading suppliers allow: – 50% MOQ reduction when bundling with chairs/autoclaves – Quarterly cumulative MOQs (e.g., 12 units over 12 months) |
Structure agreements around annual volume commitments to access distributor-tier pricing without single-batch pressure. |
Step 3: Shipping & Logistics – DDP vs. FOB Analysis
2026 Incoterms® 2020 compliance is non-negotiable. Critical considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Buyer controls freight costs • Potential 12-18% savings via preferred carriers • Requires in-house logistics expertise |
• Title/risk transfers at port • Buyer liable for: – Port congestion delays (avg. 72hrs Shanghai 2025) – Customs clearance errors – Last-mile damage |
Only for distributors with: – Dedicated import compliance team – $500k+ annual shipment volume – Established carrier relationships |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (quoted in destination currency) • 5-9% premium vs FOB • No hidden port fees |
• Supplier bears: – Export/import compliance – Customs brokerage – In-transit insurance • Single-point accountability |
Recommended for 83% of clinics/distributors Essential for first-time importers. Verify supplier’s DDP experience via: – Past shipment documentation – Local agent credentials in your country |
Verified Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Strategic Value Proposition for 2026 Sourcing:
- Certification Integrity: ISO 13485:2016 (Certificate #CN-184296) & CE MDR 2017/745 (NB 2797) with full technical file access. EUDAMED listed since Q3 2025.
- MOQ Flexibility: 8-unit MOQ for intraoral X-ray systems (models CJ-5200/CJ-5500), 5-unit for bundled orders with dental chairs. OEM at 12 units with no setup fees.
- DDP Excellence: 99.2% on-time delivery (2025 data) with destination-port DDP quotes inclusive of:
– EU/US customs clearance
– Local compliance certification (e.g., Health Canada, ANVISA)
– 12-month logistics insurance - Technical Assurance: In-house sensor calibration lab (ISO/IEC 17025 accredited), 19 years continuous export history to 87 countries.
Direct Procurement Channel:
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Reference “2026 Dental Sourcing Guide” for priority DDP quotation & factory audit scheduling
Conclusion: 2026 Sourcing Imperatives
Successful intraoral X-ray procurement from China requires:
• Proactive certification validation beyond document review
• MOQ structuring aligned with volume commitment capabilities
• DDP as default for risk-averse supply chains
Partnering with established manufacturers like Shanghai Carejoy – with verifiable regulatory infrastructure and 19 years of export compliance – mitigates 74% of common sourcing failures (2025 Dental Distributor Survey). Always demand factory audit access and EUDAMED/FDA listing verification before PO issuance.
© 2026 Global Dental Procurement Consortium | This guide is for informational purposes only. Verify all regulatory requirements with local authorities. Shanghai Carejoy Medical Co., LTD is presented as an industry-verified case study based on 2025 supply chain audit data.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Intraoral X-Ray Machines – Key Procurement FAQ (2026 Edition)
Frequently Asked Questions: Buying an Intraoral X-Ray Machine in 2026
| Question | Professional Insight & Recommendation |
|---|---|
| 1. What voltage requirements should I verify before purchasing an intraoral X-ray unit for my clinic in 2026? | Most modern intraoral X-ray machines operate on standard 100–240V AC, 50/60 Hz, making them suitable for global use with auto-switching power supplies. However, always confirm the specific voltage compatibility with your regional electrical infrastructure. Units intended for high-frequency digital sensors may require stable voltage input; we recommend using a line conditioner or uninterruptible power supply (UPS) to prevent damage from voltage fluctuations. Consult the technical datasheet and involve a certified dental electrician during site assessment. |
| 2. Are spare parts for intraoral X-ray machines readily available, and what components are most commonly replaced? | Reputable manufacturers maintain spare parts inventories for at least 7–10 years post-discontinuation. Commonly replaced components include X-ray tubes, position-indicating devices (PIDs), control panel buttons, cables, and collimators. When purchasing, confirm with the supplier the availability of spare parts and lead times. Distributors should ensure access to an authorized service depot. Opt for brands with established local or regional support networks to minimize downtime. |
| 3. What does the installation process involve, and is professional setup required? | Yes, professional installation by a certified technician is mandatory. The process includes mechanical mounting (wall-arm or floor stand), electrical connection, radiation safety compliance checks, and calibration. In 2026, many units integrate with clinic management software and require network configuration. Ensure your facility meets structural requirements (e.g., wall load capacity) and radiation shielding standards. Installation should be followed by a compliance certificate issued by the technician, required for regulatory audits. |
| 4. What warranty coverage should I expect from a new intraoral X-ray machine in 2026? | Standard warranty terms in 2026 range from 2 to 3 years comprehensive coverage, including parts, labor, and X-ray tube. Advanced digital models may offer extended warranties up to 5 years with optional service contracts. Verify if the warranty is global or region-locked and whether it requires scheduled maintenance to remain valid. Distributors should provide warranty registration support and direct access to technical service teams. |
| 5. How do I ensure long-term serviceability and support after the warranty expires? | Prioritize manufacturers with a documented service lifecycle policy. In 2026, leading brands offer post-warranty service agreements (PWSA), preventive maintenance plans, and firmware updates for digital integration. Confirm that software updates are included or available at a reasonable cost. Distributors should maintain spare parts logistics and certified field engineers. Request a service level agreement (SLA) outlining response times and repair turnaround for business continuity planning. |
Note: Regulations and technical standards may vary by country. Always consult local dental regulatory bodies and certified biomedical engineers before procurement and installation.
Need a Quote for Intraoral X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


