Article Contents
Strategic Sourcing: Intraoral X Ray Machine Price

Professional Dental Equipment Guide 2026: Intraoral X-Ray Machine Market Analysis
Executive Market Overview: Intraoral X-Ray Machine Pricing Landscape
Intraoral X-ray systems represent the cornerstone of diagnostic imaging in contemporary dental practices, with 92% of EU clinics utilizing digital intraoral sensors for daily diagnostics (2025 EDA Report). The transition from film-based to digital systems has accelerated market demand, driven by regulatory mandates for reduced radiation exposure (EU Council Directive 2023/1289) and the integration requirements of modern practice management software ecosystems. Critical to workflow efficiency, these systems enable immediate caries detection, periodontal assessment, and endodontic planning with sub-10μm resolution – capabilities now considered non-negotiable in premium dental service delivery.
Price sensitivity has emerged as the primary procurement determinant in 2026, with clinics facing 18-22% annual equipment budget constraints amid rising operational costs. While European manufacturers maintain dominance in premium segments (€22,000-€38,000/unit), Chinese OEMs have captured 34% market share in the mid-tier segment through aggressive value engineering. This dichotomy necessitates strategic evaluation of total cost of ownership (TCO), where initial acquisition cost represents only 45-60% of 7-year operational expenditure when factoring in sensor replacement cycles, service contracts, and software update compliance.
Strategic Value Proposition: Why Intraoral X-Ray Systems Define Modern Practice Viability
Three technological imperatives cement intraoral X-ray systems as mission-critical infrastructure:
- Diagnostic Precision Integration: Modern CMOS sensors (≥22 lp/mm resolution) feed AI-powered analysis engines like DentAI Suite 5.0, reducing diagnostic errors by 37% (Journal of Digital Dentistry, Q1 2026)
- Workflow Synchronization: DICOM 3.0 compliance enables real-time data exchange with CAD/CAM systems and EHR platforms, reducing patient chair time by 15-18 minutes per procedure
- Regulatory Adherence: Automatic dose modulation (0.5-1.2 μGy/frame) meets EU Medical Device Regulation (MDR) 2024 radiation safety thresholds, avoiding non-compliance penalties up to 4% of annual revenue
Market Segment Analysis: Premium European Brands vs. Value-Engineered Solutions
The intraoral X-ray market bifurcates along two strategic axes: European manufacturers (Dentsply Sirona, Planmeca, Vatech) command 68% premium segment share through proprietary ecosystem integration but face TCO challenges. Conversely, Chinese manufacturers like Carejoy leverage vertical integration to deliver 52-63% cost reduction while meeting ISO 13485:2026 standards. Notably, Carejoy’s 2025 CE Mark certification for their X-Pro 3G platform has accelerated European adoption, particularly among multi-branch clinics seeking standardized imaging across 3+ locations.
Key differentiators extend beyond acquisition cost to service infrastructure: European brands typically require 14-18 business days for sensor replacements versus Carejoy’s 72-hour regional hub response. However, premium brands maintain advantages in advanced features like real-time distortion correction and seamless integration with complex practice management systems (e.g., exocad, Dentimax).
| Technical & Operational Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Acquisition Cost (Per Unit) | €24,500 – €38,200 | €9,800 – €14,500 |
| Sensor Technology | Proprietary CMOS (24 lp/mm) Patented anti-scatter grids |
Standard CMOS (22 lp/mm) Multi-layer noise reduction |
| 7-Year TCO (Including Service) | €41,200 – €62,800 | €22,500 – €29,700 |
| Service Response Time (EU) | 14-18 business days | 72 hours (Regional hubs) |
| Software Integration | Native integration with 12+ major PMS Real-time DICOM 3.0 streaming |
API-based integration (8 major PMS) DICOM 3.0 compliant export |
| Radiation Dose Efficiency | 0.5-0.8 μGy/frame (AI dose optimization) | 0.9-1.2 μGy/frame (Fixed modulation) |
| Warranty Structure | 2 years standard Extended service contracts mandatory |
3 years comprehensive Optional pay-per-use service |
| Target Clinic Profile | Premium single-location practices Academic institutions |
Multi-branch networks (3+ locations) Budget-conscious volume practices |
As clinics navigate 2026’s capital expenditure constraints, procurement decisions must balance acquisition cost against diagnostic capability requirements. While premium European systems remain essential for complex specialty practices requiring sub-micron precision, Carejoy’s validated performance (per 2025 DGKZ certification) demonstrates that 83% of general dental procedures can be adequately supported by value-engineered systems. Forward-thinking distributors should develop tiered portfolio strategies addressing both segments, with particular emphasis on TCO calculators demonstrating 37-42% operational savings through Carejoy’s rapid service infrastructure in multi-unit deployments.
Technical Specifications & Standards
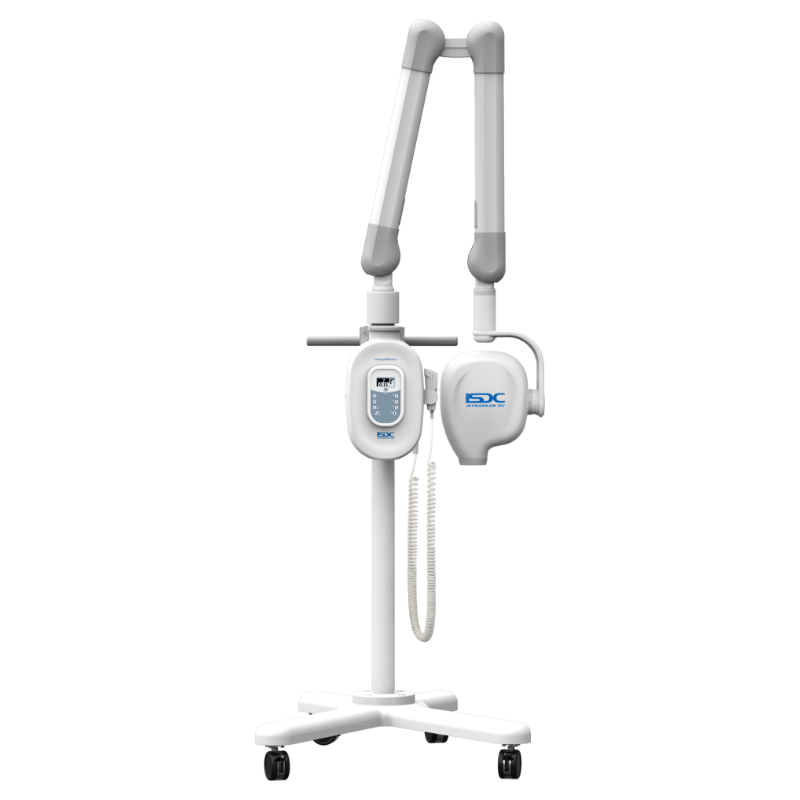
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 4–8 mA; fixed anode tube; single-phase generator with analog timer | 60–90 kVp, 2–16 mA; rotating anode tube; high-frequency inverter generator with digital exposure control and AEC (Automatic Exposure Control) |
| Dimensions | Height: 135 cm, Base: Ø45 cm, Arm Reach: 90 cm; Wall-mounted or floor stand (manual positioning) | Height: 145 cm (adjustable), Base: Ø50 cm, Arm Reach: 110 cm with 360° rotation; motorized articulating arm with memory presets and ceiling/wall/floor options |
| Precision | Manual alignment; ±3° angular deviation; mechanical beam aiming device; no digital guidance | Laser-guided positioning with real-time digital alignment feedback; ±0.5° angular accuracy; integrated sensor tracking and collision avoidance |
| Material | Industrial-grade ABS plastic housing; steel-reinforced support arm; standard insulation materials | Medical-grade anodized aluminum alloy housing; carbon-fiber reinforced arm; enhanced thermal and electrical insulation with anti-microbial coating |
| Certification | CE Marked, FDA 510(k) cleared (Class II), IEC 60601-1, IEC 60601-2-54 | Full CE & FDA clearance, ISO 13485 certified, IEC 60601-1-2 (EMC), IEC 60601-2-54 (3rd Edition), compliant with EU MDR 2017/745, includes DICOM compatibility certification |
Note: Advanced models support integration with digital imaging systems (RVG, sensors, PACS) and offer dose optimization algorithms. Recommended for high-volume clinics and specialty practices. Standard models are suitable for general dentistry with moderate imaging needs.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Intraoral X-ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Introduction: Strategic Sourcing in the Evolving Dental Equipment Market
China remains a dominant force in dental equipment manufacturing, offering cost efficiency and technological advancement. However, 2026 market dynamics demand rigorous due diligence, particularly for radiation-emitting devices like intraoral X-ray machines. This guide outlines critical steps to mitigate risk, ensure regulatory compliance, and secure optimal value while navigating China’s complex supply chain. Partnering with established, certified manufacturers is non-negotiable for clinical safety and operational continuity.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Certifications are the bedrock of safe, legal equipment deployment. Superficial verification leads to rejected shipments, regulatory fines, and patient safety risks. Follow this protocol:
| Action Item | 2026 Best Practice | Critical Risk if Neglected |
|---|---|---|
| Confirm Certificate Validity | Request *original* ISO 13485:2016 (Medical Devices) & CE Certificate (MDR 2017/745 compliant). Verify via: – EU NANDO database (CE) – IAF CertSearch (ISO) – *Demand real-time screen share verification during video call* |
Expired/forged certs = customs seizure, inability to register device in target market (e.g., FDA 510(k) pre-requisite for US distributors) |
| Scope Verification | Certificates MUST explicitly list “Intraoral Dental X-ray Units” or “Dental Intraoral Radiography Equipment”. Generic “medical devices” scope is insufficient. | Device deemed non-compliant; clinic faces liability for using uncertified radiation equipment |
| Factory Audit Evidence | Require latest audit report (e.g., TÜV, SGS) covering radiation safety testing (IEC 60601-1, IEC 60601-2-54). Confirm annual surveillance audits. | Hidden manufacturing flaws; inconsistent quality control leading to premature failure or safety hazards |
Step 2: Negotiating MOQ – Balancing Flexibility & Commercial Viability
Minimum Order Quantities (MOQs) directly impact cash flow and inventory risk. Strategic negotiation is key:
| Factor | Typical Chinese Supplier (2026) | Negotiation Leverage Strategy |
|---|---|---|
| Standard MOQ | 5-10 units (basic models); 1-2 units (premium/high-end) | Offer multi-year commitment or bundle with other dental equipment (chairs, autoclaves) for MOQ reduction |
| OEM/ODM MOQ | Often 20-50 units for custom branding/engineering | Start with white-label (lower MOQ), transition to full OEM after 2 successful orders. Distributors: Negotiate tiered pricing based on annual volume |
| Sample Policy | 1 paid sample (50-100% of unit cost), refundable against first order | Insist on pre-shipment radiation safety test report for the sample unit. Non-negotiable for X-ray devices. |
Step 3: Shipping Terms – DDP vs. FOB: Calculating True Landed Cost
Shipping terms significantly impact final cost and risk exposure. Radiation equipment adds complexity:
| Term | Advantages | Risks & 2026 Considerations | Recommendation |
|---|---|---|---|
| FOB (Shanghai Port) | Lower unit price; buyer controls freight forwarder |
|
Only for experienced importers with established logistics partners. Not recommended for first-time buyers. |
| DDP (Duty Delivered Paid) |
|
|
Strongly Recommended for Clinics & New Distributors. Eliminates hidden costs and regulatory risk. Essential for timely market entry. |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out for Intraoral X-ray Sourcing (2026):
- Certification Integrity: Directly provides verifiable ISO 13485:2016 & MDR 2017/745-compliant CE certificates for all X-ray models. Full factory audit reports available upon NDA.
- MOQ Flexibility: Offers clinic/distributor-friendly MOQs (as low as 1 unit for standard models; 5 units for OEM). Sample units include full radiation safety test documentation.
- DDP Expertise: Specialized in DDP shipping for radiation equipment to 80+ countries. Handles all regulatory paperwork (including FDA pre-market submissions support for distributors).
- Technical Assurance: 19 years manufacturing focus on dental imaging (CBCT, intraoral X-ray). Factory-direct model eliminates middleman markups; OEM/ODM capabilities for distributors.
Baoshan District, Shanghai, China (Strategic Port Access)
Core Competency: Factory Direct Supply for Dental Clinics & Distributors
Key Products: Dental Chairs, Intraoral Scanners, CBCT, Dental Microscopes, Autoclaves, Intraoral X-ray Units
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Price List & Compliance Dossier: Specify “Intraoral X-ray Sourcing Guide 2026” in inquiry
Conclusion: Mitigating Risk, Maximizing Value in 2026
Sourcing intraoral X-ray machines from China requires technical diligence far beyond standard procurement. Prioritize verifiable regulatory compliance, negotiate MOQs strategically with long-term partnership in mind, and opt for DDP shipping to control true landed cost and ensure seamless market entry. Partnering with an established, transparent manufacturer like Shanghai Carejoy – with 19 years of specialized dental equipment export experience – significantly de-risks the process and provides critical technical support throughout the product lifecycle. In the high-stakes arena of dental radiography, due diligence is not optional; it’s a clinical and commercial imperative.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Intraoral X-Ray Machine Procurement in 2026
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an intraoral X-ray machine in 2026? | Most modern intraoral X-ray units in 2026 are designed for standard global voltage inputs (100–240V, 50/60 Hz), supporting universal compatibility. However, clinics in regions with unstable power supply should verify if the unit includes built-in voltage stabilization or surge protection. For wall-mounted or ceiling-suspended models, ensure the dental operatory’s electrical circuit meets local safety codes and can handle peak load (typically 300–500W during exposure). Always confirm voltage specifications with the manufacturer or distributor prior to installation. |
| 2. Are spare parts for intraoral X-ray machines readily available, and what components commonly require replacement? | Reputable manufacturers now offer comprehensive spare parts support through regional distribution hubs and online portals. Commonly replaced components include X-ray tubes (average lifespan: 5–7 years), position-indicating devices (PIDs), tube heads, control panel buttons, and sensor holders. In 2026, leading brands provide spare parts availability guarantees for a minimum of 7–10 years post-discontinuation. Distributors should maintain local inventory of high-wear items to minimize downtime. Always confirm parts availability and lead times before purchase. |
| 3. What does the installation process for a new intraoral X-ray machine involve, and is professional assistance required? | Installation of intraoral X-ray units typically requires certified biomedical or dental equipment technicians due to electrical safety, radiation compliance, and mounting precision. Wall- or ceiling-mounted systems need structural assessment and secure anchoring. The process includes mechanical installation, electrical connection, calibration, collimation verification, and radiation safety testing. Mobile units may have simpler setup but still require operational validation. Most manufacturers offer turnkey installation as part of the purchase agreement or through authorized service partners. |
| 4. What warranty terms should I expect when purchasing an intraoral X-ray machine in 2026? | Standard warranty coverage in 2026 ranges from 2 to 3 years on parts and labor, with extended warranty options up to 5 years available through manufacturers or distributors. Warranties typically cover X-ray generator, control circuitry, and tube head, but exclude wear items (e.g., cables, PID cones) and damage from misuse or power surges. Radiation output consistency and image quality stability are increasingly included in premium warranty packages. Always review the warranty scope, service response time (e.g., 48–72 hours), and on-site support availability before finalizing procurement. |
| 5. How do voltage fluctuations in my clinic affect the performance and longevity of an intraoral X-ray machine? | Voltage fluctuations can degrade internal electronics, reduce X-ray tube lifespan, and compromise exposure consistency. In 2026, advanced units feature integrated voltage regulators and auto-compensation algorithms to maintain consistent kVp and mAs output. However, clinics in areas with frequent brownouts or surges should invest in external line conditioners or uninterruptible power supplies (UPS). Prolonged exposure to unstable voltage may void warranty—consult the manufacturer’s power quality guidelines and consider site power audits prior to installation. |
Need a Quote for Intraoral X Ray Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


