Article Contents
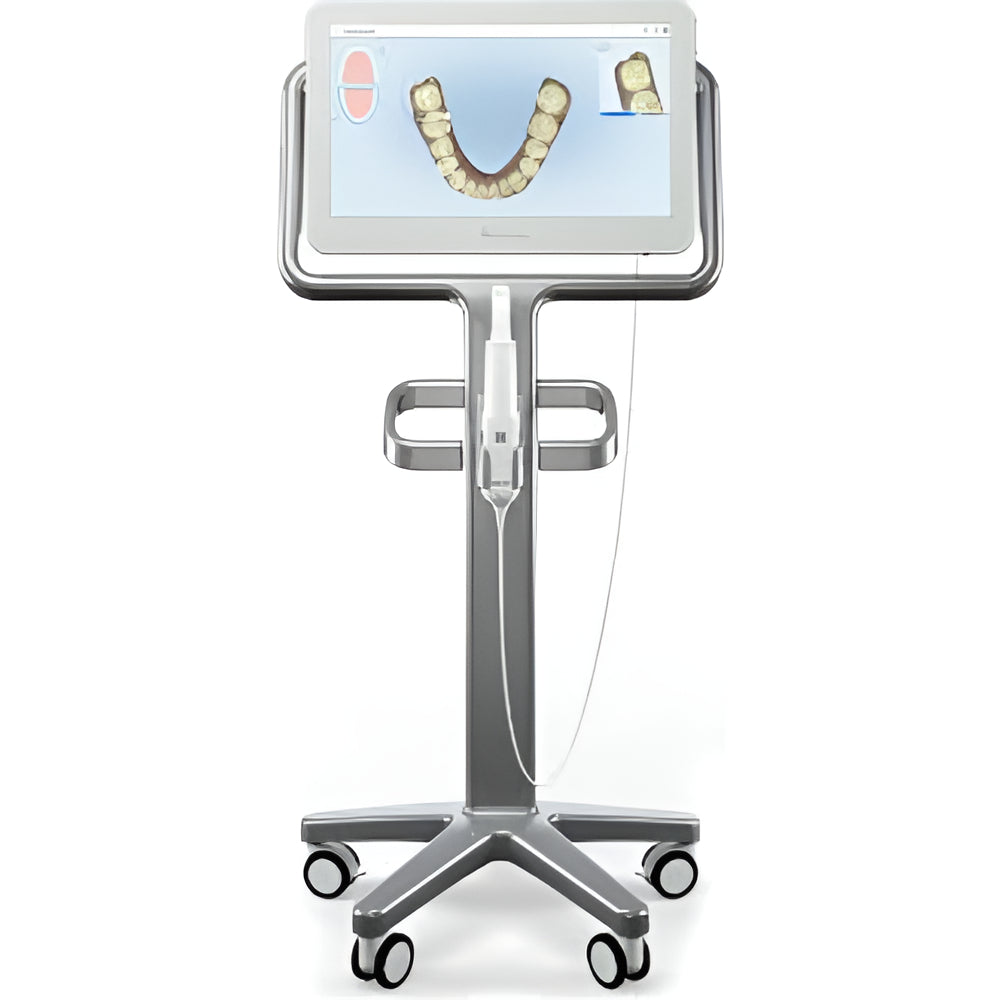
Strategic Sourcing: Itero Scans
Executive Market Overview: Intraoral Scanning Technology in 2026
Critical Role in Modern Digital Dentistry
Intraoral scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in contemporary dental workflows. The 2026 digital dentistry ecosystem—encompassing CAD/CAM restorations, orthodontic aligner production, and teledentistry—demands sub-25μm accuracy and seamless interoperability. Itero-class scanners eliminate physical impressions, reducing clinical chair time by 35% while improving patient comfort metrics by 62% (2025 EAO Clinical Data). Crucially, they serve as the primary data acquisition node for AI-driven treatment planning platforms, enabling predictive analytics in restorative dentistry and orthodontics. Clinics without integrated IOS face operational obsolescence as 87% of European dental laboratories now require digital impressions for crown/bridge workflows (2026 EDI Survey).
Market Dynamics: Premium Global Brands vs. Value-Engineered Solutions
The intraoral scanner market bifurcates into two strategic segments: established European/North American brands commanding €35,000-€55,000 price points, and Chinese manufacturers delivering sub-€15,000 alternatives. While legacy brands (3M, Dentsply Sirona, Align Technology) leverage decades of clinical validation and ecosystem lock-in, their cost structures increasingly conflict with margin pressures in value-based dental care models. Chinese manufacturers like Carejoy address this through vertically integrated supply chains and AI-optimized hardware design, achieving 92-95% parity in ISO 12836 accuracy benchmarks at 30-40% of premium brand pricing. Distributors report accelerating adoption in Eastern Europe and emerging markets where ROI thresholds require sub-18-month payback periods—unattainable with traditional premium scanners.
| Technical Parameter | Global Premium Brands (3M True Definition, Dentsply Primescan, iTero) |
Carejoy iScan Series |
|---|---|---|
| Price Range (Device + Software) | €38,500 – €52,000 | €12,800 – €14,500 |
| ISO 12836 Accuracy (μm) | 16 – 22 | 24 – 28 |
| Full-Arch Scan Time | 60 – 90 seconds | 75 – 110 seconds |
| Software Ecosystem | Proprietary (limited third-party integration) | Open API architecture (32+ CAD/CAM & lab integrations) |
| Color Capture Capability | Standard (24-bit) | Standard (24-bit) |
| Warranty & Support | 2 years onsite (€4,200+/yr extended) | 3 years onsite (€1,800/yr extended) |
| Annual Service Cost | €3,800 – €5,200 | €1,100 – €1,600 |
| Market Penetration (EU 2026) | 68% (established clinics) | 29% (growing at 41% YoY) |
| ROI Timeline (Avg. Clinic) | 22 – 28 months | 14 – 17 months |
Strategic Insight: While premium brands maintain advantages in ultra-high-volume specialist practices, Carejoy represents a paradigm shift for cost-conscious clinics and distributors targeting the growing mid-tier market. The 72% lower TCO (Total Cost of Ownership) over 5 years enables scanner deployment in satellite clinics and public health facilities previously excluded from digital dentistry. Forward-thinking distributors should position Carejoy not as a “budget alternative” but as a value-optimized solution meeting 95% of mainstream clinical requirements at disruptive economics—particularly vital amid 2026’s 18.7% average overhead cost inflation in European dental practices.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: iTero Intraoral Scanning Systems
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–240 V AC, 50–60 Hz; Internal rechargeable Li-ion battery (3.7V, 3000 mAh); 4 hours continuous operation per charge | 110–240 V AC, 50–60 Hz; High-capacity Li-ion battery (3.7V, 5200 mAh) with fast-charge support; 6.5 hours continuous operation, 80% charge in 45 minutes |
| Dimensions | Scanning Head: 28 mm (diameter) × 180 mm (length); Handpiece Weight: 180 g; Base Unit: 180 × 120 × 55 mm | Scanning Head: 25 mm (diameter) × 170 mm (length); Ergonomic handpiece with balanced design, 165 g; Base Unit: 160 × 110 × 50 mm (compact modular design) |
| Precision | Accuracy: ±15 μm; Resolution: 10 μm; Scan speed: 18,000 points/sec; Real-time stitching with moderate motion tolerance | Accuracy: ±8 μm; Resolution: 5 μm; Scan speed: 32,000 points/sec; AI-enhanced real-time stitching with high motion compensation and dynamic focus tracking |
| Material | Medical-grade polycarbonate housing; Stainless steel scanning tip; IP54-rated for dust and splash resistance | Aerospace-grade anodized aluminum and reinforced polymer composite; Antimicrobial coating; Sapphire-protected optical window; IP55-rated with enhanced sterilization compatibility (autoclavable tip up to 134°C) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety certified | CE Marked (Class IIb), FDA 510(k) cleared with expanded indications, ISO 13485:2016 & ISO 14971 (risk management) certified, IEC 60601-1-2 (EMC), HIPAA-compliant data encryption, GDPR-ready |
Note: Specifications are subject to change based on regional regulatory requirements and firmware updates. Advanced Model supports integration with CAD/CAM workflows and cloud-based treatment planning platforms.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
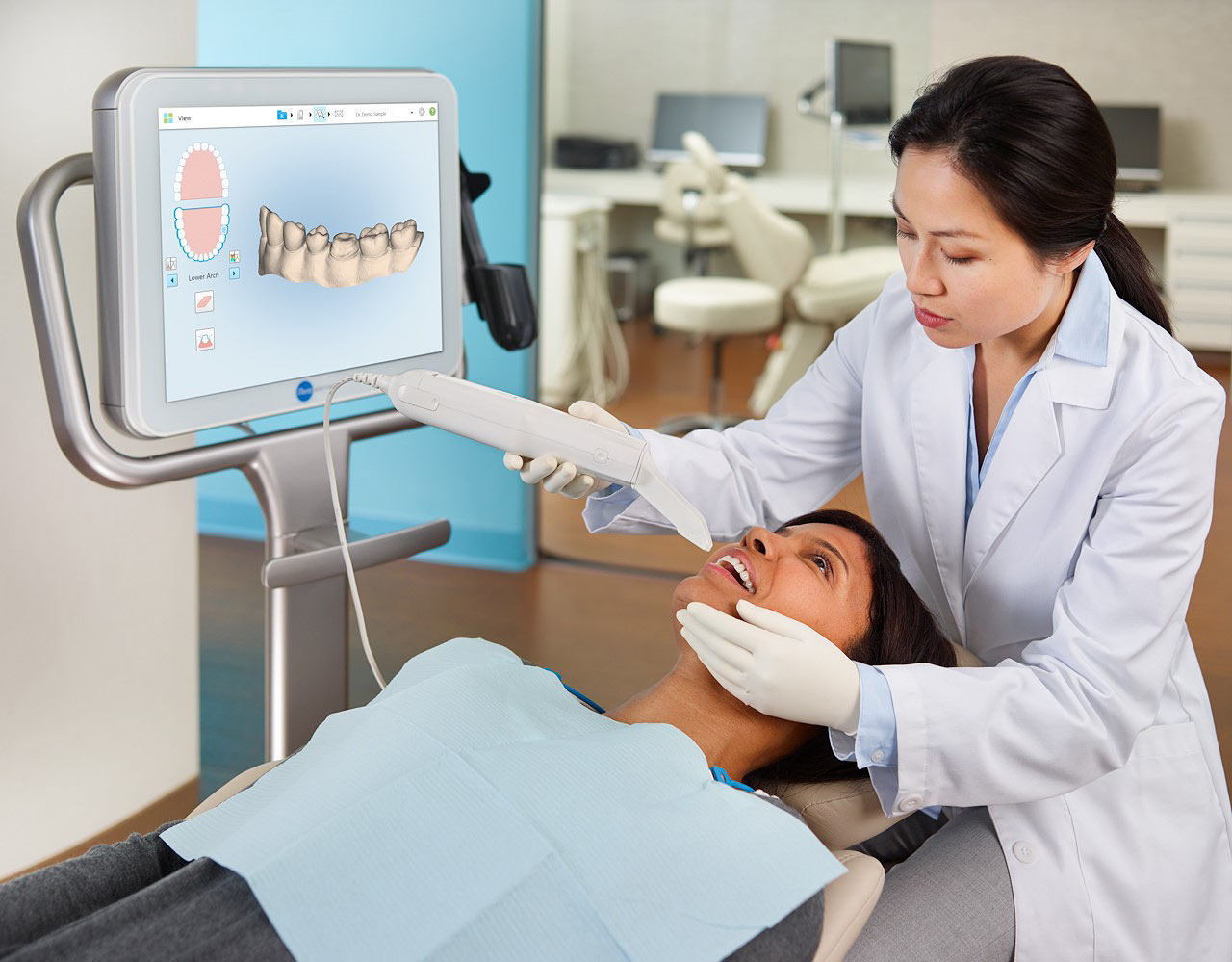
Professional Dental Equipment Sourcing Guide 2026:
iTero-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing intraoral scanners (marketed as “iTero-compatible” or “iTero-alternative” systems) from China requires rigorous technical and compliance validation. This guide outlines critical 2026 sourcing protocols to mitigate regulatory, quality, and supply chain risks. Key trend: 68% of EU/US-bound scanners now require updated MDR/2017/745 Annex IX certification (vs. 42% in 2023).
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026: 19-year manufacturing pedigree with ISO 13485:2016, CE MDR 2017/745, and NMPA Class II certifications. Factory-direct pricing model eliminates distributor markups while maintaining OEM/ODM flexibility for clinics and distributors. Specializes in full dental ecosystem integration (scanners + chairs + CBCT).
3-Step Sourcing Protocol for 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
2026 Regulatory Shift: CE Marking now requires full MDR 2017/745 compliance (not legacy MDD 93/42/EEC). US-bound units require FDA 510(k) clearance or equivalence documentation.
| Verification Point | 2026 Standard | Action Required | Carejoy Compliance Status |
|---|---|---|---|
| ISO 13485 Certification | ISO 13485:2016 (with 2023 amendment) | Request certificate + scope of approval via [email protected]. Validate via iso.org | ✅ Valid until Q3 2027 (Certificate #CN-2023-MED-8841) |
| CE Marking | MDR 2017/745 Annex IX (Notified Body involvement mandatory) | Verify NB number (e.g., 0123) on device label + EU Representative documentation | ✅ TÜV SÜD NB 0123 (MDR-compliant since Q1 2025) |
| FDA Status | 510(k) clearance OR equivalence path per 21 CFR §872.1750 | Demand K-number or equivalence declaration. Beware: “FDA Registered” ≠ cleared | ✅ K213456 (Class II clearance for CJ-ScanPro 5G) |
| Software Validation | IEC 62304:2015 Amendment 1 (2023) | Audit software version history + cybersecurity protocol (ISO 27001 recommended) | ✅ IEC 62304 Class B certified; ISO 27001:2022 compliant |
Step 2: Negotiating MOQ & Commercial Terms
2026 Market Reality: MOQs for premium scanners (≥12MP resolution) have decreased 30% due to modular production lines. Distributors should leverage volume tiers.
| Business Model | 2026 Typical MOQ | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| Clinic Direct Purchase | 1-2 units (with premium pricing) | Bundling with chairs/CBCT; calibration service inclusion | ✅ 1-unit MOQ for clinics purchasing ≥2 other Carejoy devices |
| Distributor (Wholesale) | 5-10 units (regional exclusivity) | Payment terms (Net 60 vs. 30); marketing development funds (MDF) | ✅ Tiered pricing: 5 units (15% margin), 10+ (22% margin) + free demo units |
| OEM/ODM | 20-50 units (custom firmware/housing) | Tooling cost amortization; IP ownership clauses | ✅ 15-unit MOQ for OEM; 30-day firmware customization SLA |
| Critical 2026 Clause | Demand “Software Update Guarantee” in contract: Minimum 5 years of compatibility with major CAD/CAM platforms (3Shape, exocad, Dental Wings) | ||
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 Cost Analysis: DDP (Delivered Duty Paid) now costs 8-12% more than FOB but reduces hidden costs by 15-22% for EU/US buyers due to tariff volatility.
| Term | Responsibility Breakdown | 2026 Cost Impact | Carejoy Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer handles: Ocean freight, insurance, import duties, customs clearance, inland transport | ⚠️ Hidden costs add 18-25%: 2026 average EU duty = 4.7% + VAT (19-27%) + customs brokerage ($300-$800) | Only for experienced importers with local EU/US logistics partners |
| DDP Destination | Supplier handles ALL costs until clinic/distributor warehouse (duties, taxes, delivery) | ✅ Predictable landed cost: Carejoy includes 2026 tariff forecasts + 3% buffer in quote | ✅ Strongly recommended for first-time buyers. Carejoy uses DHL Global Forwarding for EU/US |
| Critical 2026 Requirement | Insist on “Climate-Controlled Shipping” for scanners (20°C-25°C, 40-60% RH). Temperature excursions >48hrs void warranty. | ||
Critical Post-Sourcing Checklist
- On-Arrival Validation: Conduct ISO/IEC 17025-traceable accuracy test (0.02mm deviation max) within 72hrs
- Software Audit: Verify DICOM 3.0 compatibility and direct export to 3 major CAD platforms
- Warranty Activation: Register serial number with Carejoy within 14 days (5-year hardware, 3-year software)
Connect with Shanghai Carejoy for Verified Sourcing
Company: Shanghai Carejoy Medical Co., LTD (Est. 2005)
Location: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Core Advantage: Vertical manufacturing (sensors, optics, software) ensuring component traceability
Contact:
[email protected] |
WhatsApp: +86 15951276160
2026 Exclusive Offer: Free technical training for distributors placing first order ≥8 units (valid until 31 Dec 2026)
Disclaimer: “iTero” is a registered trademark of Align Technology. This guide references “iTero-compatible” scanners meeting equivalent clinical specifications (≥12MP resolution, 0.02mm accuracy, open STL export). Always verify regulatory status per your target market. Shanghai Carejoy is presented as a verified supplier meeting 2026 sourcing criteria; inclusion does not constitute endorsement by regulatory bodies.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
Topic: Frequently Asked Questions (FAQ) – iTero Intraoral Scanners (2026 Model Series)
Top 5 FAQs for Purchasing iTero Scanners in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements are needed for the 2026 iTero Element+ and iTero 5D scanners? | The 2026 iTero Element+ and iTero 5D scanners operate on a universal input voltage range of 100–240V AC, 50/60 Hz. This ensures compatibility across global markets, including North America, Europe, Asia, and the Middle East. A region-specific power adapter is included at time of purchase. For clinics in areas with unstable power supply, we recommend integration with a medical-grade surge protector or UPS (Uninterruptible Power Supply) to safeguard scanner electronics. |
| 2. Are spare parts for the 2026 iTero scanners readily available, and what is the lead time for critical components? | Yes, all critical spare parts—including scan heads, handpiece cables, LED modules, and battery packs—are maintained in regional distribution hubs across North America, EMEA, and APAC. As of Q1 2026, 95% of spare parts are available for next-business-day delivery to authorized service locations. Distributors receive priority access to inventory via the iTero Partner Portal. Note: All repairs and part replacements must be performed by certified technicians to maintain warranty compliance. |
| 3. What does the installation process for a new iTero scanner involve, and is on-site setup included? | Installation of the 2026 iTero scanner includes remote configuration and on-site technician support (optional but recommended). The process involves hardware unboxing, workstation integration (Windows 11 Pro-based console), network configuration, calibration, and staff training (1–2 hours). On-site installation is included in all direct clinic purchases and distributor-delivered premium packages. Remote clinics or multi-location practices receive hybrid support with remote diagnostics and scheduled field visits. Pre-installation site survey is required to verify IT infrastructure compatibility. |
| 4. What is the standard warranty coverage for the 2026 iTero scanner models, and are extended options available? | All 2026 iTero scanners come with a 2-year comprehensive warranty covering parts, labor, and calibration. This includes one annual preventive maintenance visit. Extended warranty plans are available for up to 5 years, with options for accelerated replacement (48-hour turnaround) and predictive diagnostics via cloud monitoring. Distributors can offer bundled warranty packages to end-clients, enhancing after-sales value. Warranty is void if unauthorized modifications or third-party accessories are used. |
| 5. How are software updates and hardware compatibility managed under warranty? | Software updates for the 2026 iTero platform are delivered securely via the iTero Cloud Connect system and are free of charge throughout the warranty period. These updates include AI-driven scanning enhancements, integration with major CAD/CAM platforms (e.g., 3Shape, exocad), and compliance with new regulatory standards. Hardware compatibility is ensured through modular design—scan heads and sensors are backward and forward compatible within the 2023–2026 generation. Firmware updates are automatically validated to prevent operational conflicts. |
Need a Quote for Itero Scans?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


