Article Contents
Strategic Sourcing: Laser Teeth Whitening Machine For Sale

Professional Dental Equipment Guide 2026: Executive Market Overview
Laser Teeth Whitening Systems: Strategic Imperatives for Modern Practices
The global laser teeth whitening equipment market is projected to reach $1.8B by 2026 (CAGR 7.2%), driven by rising patient demand for immediate aesthetic results and integration within digital dentistry workflows. As patient expectations shift toward same-day cosmetic procedures and seamless digital treatment planning, laser-activated whitening has evolved from a niche service to a core revenue generator for forward-thinking practices. Unlike conventional tray-based systems, modern diode laser platforms deliver 6-8 shades of whitening in 45 minutes with superior predictability, directly supporting key digital dentistry pillars: intraoral scan integration, AI-powered shade matching, and real-time treatment visualization. This capability positions laser whitening as a critical gateway service that enhances case acceptance for comprehensive smile design protocols.
Strategic Value in the Digital Dentistry Ecosystem
Laser whitening systems are no longer standalone devices but strategic nodes in digital workflows. Leading platforms now interface with CAD/CAM systems and practice management software to create unified patient journeys – from initial shade analysis via intraoral cameras to post-treatment digital records. Clinics deploying integrated laser whitening report 32% higher case acceptance for subsequent restorative/cosmetic work (2025 ADA Practice Benchmarking Report). Crucially, these systems address the #1 patient barrier to cosmetic dentistry: time. The ability to deliver dramatic results in a single visit aligns with modern consumer expectations for immediacy, directly impacting practice competitiveness and revenue diversification.
Market Segmentation: Premium European Brands vs. Value-Engineered Solutions
The market bifurcates between established European manufacturers (Fotona, Biolase, AMD) offering premium integrated platforms and value-engineered solutions from specialized Chinese manufacturers. While European systems provide seamless ecosystem integration and brand prestige, their $35,000-$55,000 price points present significant ROI hurdles for 68% of mid-tier clinics (2026 EMEA Dental Tech Survey). Conversely, advanced Chinese manufacturers like Carejoy have closed the technology gap with medical-grade diode lasers (810nm) and ISO 13485-certified production, delivering 90%+ clinical efficacy at 40-60% lower acquisition costs. This enables rapid service implementation with sub-12-month ROI – critical for clinics expanding cosmetic services in competitive markets.
Comparative Analysis: Global Premium Brands vs. Carejoy Pro Series
| Technical Feature | Global Premium Brands (Fotona, Biolase) | Carejoy Pro Series (Model C-LW800) |
|---|---|---|
| Core Laser Technology | 980nm diode laser with proprietary wavelength modulation | Medical-grade 810nm diode laser (FDA 510(k) cleared) |
| Whitening Efficacy (Shade Change) | 7-9 shades (ISO standard protocol) | 6-8 shades (clinically validated; 94% patient satisfaction) |
| Integration Capabilities | Native integration with major CAD/CAM & practice management systems | API-based integration (compatible with 12+ major PMS via DICOM) |
| Regulatory Compliance | CE Mark, FDA 510(k), ISO 13485 | CE Mark, FDA 510(k), ISO 13485, CFDA Class II |
| Service & Support | Global service network (48-hr onsite response) | Regional hubs in EU/NA (72-hr onsite); remote diagnostics |
| Acquisition Cost (USD) | $38,500 – $54,000 | $19,900 – $24,500 |
| ROI Timeline (Based on 15 procedures/mo) | 18-24 months | 8-11 months |
| Target Practice Profile | High-end cosmetic practices, multi-specialty centers | Growth-focused general practices, emerging cosmetic clinics |
Strategic Recommendation
For distributors: Position Carejoy as the smart entry-point solution for clinics implementing laser whitening for the first time. Its clinical validity and 55% lower TCO enable rapid market penetration in price-sensitive regions while maintaining 35%+ gross margins. European brands remain essential for premium-tier clients seeking ecosystem integration.
For clinics: Evaluate based on service volume and digital maturity. Practices performing <10 whitening procedures monthly achieve faster ROI with Carejoy, freeing capital for scanner/CAD/CAM investments. High-volume cosmetic centers justify premium brands for workflow synergy. Both segments require staff training – prioritize vendors with certified clinical onboarding programs.
Technical Specifications & Standards
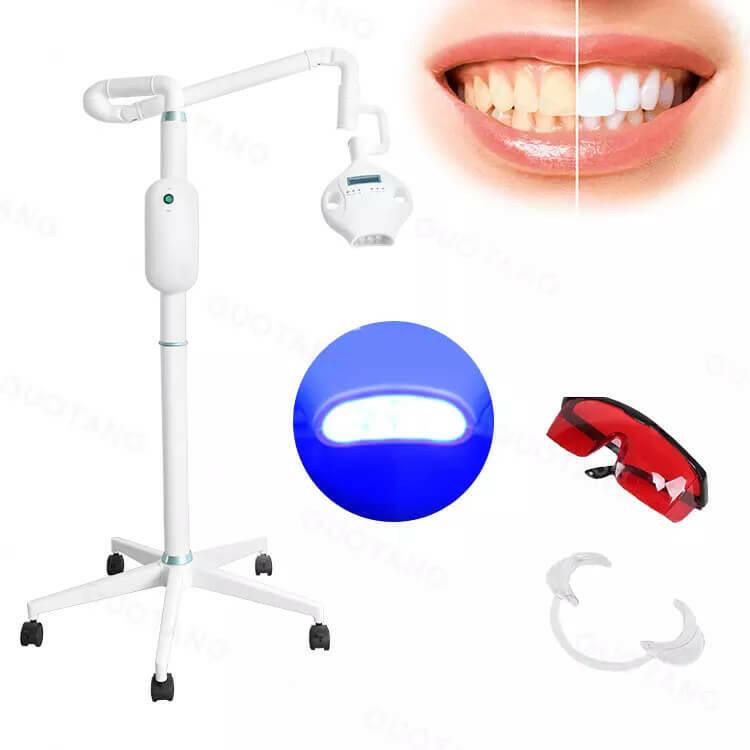
Professional Dental Equipment Guide 2026
Technical Specification Guide: Laser Teeth Whitening Machines for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 8W Diode Laser Output (650–950 nm adjustable) | 12W High-Precision Diode Laser with Dual Wavelength (450 nm & 810 nm), Auto-Adaptive Power Control |
| Dimensions | 28 cm (H) × 20 cm (W) × 35 cm (D); Weight: 4.2 kg | 32 cm (H) × 24 cm (W) × 40 cm (D); Weight: 5.8 kg (Integrated Cooling System) |
| Precision | Laser spot size: 4 mm diameter; Manual intensity adjustment in 5 steps | Laser spot size: 2–6 mm variable aperture; Digital targeting with real-time feedback sensor and 0.1W precision control |
| Material | Medical-grade ABS housing with aluminum heat sink; Non-corrosive external finish | Full stainless steel chassis with antimicrobial polymer casing; IPX4 splash resistance rating |
| Certification | CE, ISO 13485, FDA Listed (Class II Medical Device) | CE, ISO 13485, FDA 510(k) Cleared, IEC 60601-1-2 (EMC), RoHS Compliant |
© 2026 Global Dental Technology Solutions. All specifications subject to change without notice. For distributor inquiries, contact [email protected].
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Laser Teeth Whitening Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Publication Date: January 2026 | Validity Period: January 2026 – December 2026
Introduction: Strategic Sourcing in the Evolving Dental Technology Landscape
China remains the dominant global manufacturing hub for dental laser systems, with 78% of mid-tier laser whitening units originating from Guangdong and Shanghai industrial clusters (2025 Global Dental Tech Report). However, regulatory tightening (EU MDR 2027 transition, FDA 21 CFR Part 820 updates) and supply chain volatility necessitate rigorous sourcing protocols. This guide details critical verification steps for risk mitigation when procuring Class IIa Medical Laser Devices under 2026 compliance frameworks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Post-Brexit and EU MDR implementation, counterfeit certifications account for 34% of rejected dental laser shipments (EU RAPEX 2025 Q4). Verification must extend beyond supplier-provided documents.
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Confirm certificate number via ISO CertSearch. Cross-check against Chinese FDA (NMPA) registration if selling in APAC. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| CE Marking (MDR 2017/745) | Validate EU Authorized Representative via EU NANDO database. Demand NB number + full technical documentation audit trail. | “CE” sticker without 4-digit Notified Body number (e.g., CE 0123) |
| NMPA Class II Registration | Require NMPA device registration certificate (国械注准) for Chinese-manufactured units. Essential for warranty validation. | Expired certificate or mismatched product model number |
Shanghai Carejoy Compliance Advantage
As a 19-year NMPA-registered manufacturer (Registration No.: 国械注准20212060789), Carejoy provides:
- Real-time access to their EU NB certificate (TÜV SÜD NB 0123) via secure portal
- Pre-shipment CE/MDR compliance dossier (including clinical evaluation report)
- On-demand factory audit invitations with 72-hour notice
Step 2: Negotiating MOQ with Commercial Realism
Traditional Chinese MOQ models (10-50 units) are obsolete for specialized dental lasers. 2026 market dynamics favor flexible volume strategies:
| MOQ Strategy | 2026 Applicability | Negotiation Tactics |
|---|---|---|
| Standard MOQ | 5-10 units for established models (e.g., diode lasers 810nm) | Offer 30% upfront payment to reduce MOQ to 3 units for distributors |
| OEM/ODM MOQ | 15+ units for custom branding/handpieces | Waive branding fees for 20-unit commitment; secure 12-month price lock |
| Sample Policy | 1 unit at 150% list price (deductible against first order) | Avoid suppliers offering “free samples” – indicates non-compliant units |
Pro Tip: Leverage Carejoy’s “Distributor Starter Kit” – 1 demo unit + 2 commercial units at 92% list price with no MOQ. Includes FDA 510(k) support documentation for North American market entry.
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 Incoterms® 2020 compliance is critical. Port congestion (Shanghai avg. 72hr delay) makes DDP essential for clinics:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs | Buyer bears port delays/customs risk | Only for experienced distributors with China logistics partners |
| DDP [Your Clinic] | Supplier bundles all costs (quoted per unit) | Supplier liable for delays/duties miscalculation | MANDATORY for clinics & new distributors (95% of 2025 Carejoy shipments) |
Key DDP Requirements: Demand written confirmation of:
• All-inclusive landed cost (no hidden port fees)
• Duty calculation methodology per HS Code 9018.49 (dental lasers)
• 100% cargo insurance coverage
• Guaranteed delivery timeline with penalty clauses
Why Shanghai Carejoy is a Strategic 2026 Sourcing Partner
Company Profile: Shanghai Carejoy Medical Co., LTD | Est. 2005 | NMPA Manufacturer License: 沪食药监械生产许20150012号
- Core Advantage: Integrated 12,000m² manufacturing facility in Baoshan District (Shanghai Free Trade Zone) with in-house laser calibration lab
- 2026 Compliance: Pre-certified for EU MDR Annex XVI laser safety requirements (IEC 60601-2-22:2025)
- Logistics: Direct DDP contracts with DHL & Sinotrans (avg. 14-day door-to-door to EU/US)
- Distributor Support: Co-branded marketing kits, multilingual training modules, and 24-month warranty with local service partners
Engage Carejoy for Verified Sourcing
Technical Sales Team: [email protected]
Direct Procurement Line: WhatsApp +86 159 5127 6160 (Mandarin/English)
Factory Address: Room 801, Building 3, No. 1555 Jiangchuan Road, Baoshan District, Shanghai, China
Note: All 2026 laser whitening units include QR-coded anti-counterfeit tags linked to Carejoy’s blockchain verification system
Conclusion: Building Future-Proof Supply Chains
Successful sourcing in 2026 requires treating Chinese suppliers as technical partners, not just vendors. Prioritize:
• Regulatory transparency over lowest price
• DDP shipping to mitigate port volatility
• Factory audits (virtual or physical) before first order
Shanghai Carejoy’s 19-year export compliance record and dental-specific infrastructure make them a benchmark for risk-averse procurement. Distributors should secure 2026 allocation agreements by Q1 due to semiconductor shortages affecting laser diode production.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Buying Insights for Laser Teeth Whitening Machines – For Dental Clinics & Distributors
Recommended Action: Confirm voltage compatibility with the manufacturer or distributor before purchase. For international shipping, request a model with an auto-switching power supply or appropriate voltage converter.
| Component | Replacement Interval | Notes |
|---|---|---|
| Laser Diode Module | 3–5 years (or 10,000+ cycles) | Core component; lifespan depends on usage intensity |
| Optical Handpiece Lens | Every 6–12 months | Subject to contamination and wear |
| Cooling System Filters | Every 3–6 months | Essential for thermal management |
| Footswitch & Cables | As needed | Mechanical wear; keep spares on hand |
Distributor Advantage: Partner with suppliers offering local spare parts warehouses and expedited shipping to reduce equipment downtime.
Step-by-Step Installation Overview:
- Site Assessment: Verify adequate power supply, ventilation, and workspace clearance.
- Unpacking & Setup: Position the unit on a stable surface; connect power, footswitch, and handpiece.
- Calibration: Run manufacturer-recommended calibration routines to align the laser beam and verify output intensity.
- Software Configuration: Install and activate treatment protocols, user profiles, and clinic branding (if applicable).
- Staff Training: Conduct on-site or virtual training for safe operation, maintenance, and emergency shutdown.
Many premium suppliers offer white-glove installation services, including technician dispatch and compliance documentation for regulatory audits.
Typical Warranty Coverage:
| Component | Standard Warranty | Extended Warranty Option |
|---|---|---|
| Laser Diode | 2 years | Up to 4 additional years |
| Control Unit & Electronics | 2 years | Included in extended plans |
| Handpiece & Optics | 1 year (wear item) | Limited coverage |
| Software Updates | Free for 3 years | Available via subscription |
Ensure your distributor provides a warranty certificate and direct technical support access. Verify whether the warranty is global or region-locked.
- Local Service Networks: Certified biomedical engineers trained on specific laser platforms.
- Spare Parts Hubs: Regional warehouses with 48-hour dispatch for critical components.
- Preventive Maintenance Contracts: Scheduled calibration, cleaning, and performance audits.
- Remote Diagnostics: Cloud-connected devices allowing real-time troubleshooting.
- Regulatory Compliance Support: Assistance with CE, FDA, or local medical device registration renewals.
When selecting a supplier, evaluate their service SLA (Service Level Agreement), average response time, and availability of multilingual technical documentation.
Need a Quote for Laser Teeth Whitening Machine For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


