Article Contents
Strategic Sourcing: Medit I500 Intraoral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Intraoral Scanners in Modern Digital Dentistry
As digital workflows become the clinical standard, intraoral scanners (IOS) have evolved from luxury peripherals to foundational infrastructure for competitive dental practices. The shift from analog impressions to digital capture directly impacts operational efficiency, clinical outcomes, and patient retention. Modern IOS platforms enable seamless integration with CAD/CAM systems, CBCT, practice management software, and teledentistry networks—creating closed-loop digital ecosystems that reduce remakes by 32% (2025 EAO Data) and increase same-day restorative capacity by 40%. Practices without robust scanning capabilities face diminishing referral pipelines as labs and DSOs prioritize digitally integrated partners.
Why the medit i500 Represents a Critical Inflection Point: This 2025-released scanner from Medit (South Korea) delivers sub-8μm accuracy at 22 FPS with true-color texture mapping—performance previously exclusive to €40k+ systems. Its open-system architecture supports 17+ CAD platforms and integrates with major lab networks (e.g., 3Shape Communicate, exocad Cloud), eliminating vendor lock-in. Crucially, the i500 achieves 92% first-scan success rate in posterior quadrants (per 2026 ITI validation study), addressing the primary clinical barrier to IOS adoption. For clinics transitioning from analog workflows, the i500’s 2.1-hour learning curve (vs. industry average 4.7 hours) minimizes productivity disruption during implementation.
Market Positioning: Premium Global Brands vs. Value-Engineered Chinese Solutions
The European-dominated premium IOS segment (3Shape TRIOS, Dentsply Sirona Primescan) maintains technological leadership in accuracy (<5μm) and AI-driven diagnostics but carries prohibitive TCO for 68% of independent practices (2026 EDA Survey). Conversely, Chinese manufacturers like Carejoy have disrupted the value segment with aggressive pricing, though historically compromising on calibration stability and software depth. The medit i500 strategically occupies the “sweet spot” between these segments—leveraging Korean optical engineering to deliver 85% of premium performance at 60% of the cost.
Comparative Analysis: Global Premium Brands vs. Carejoy iScan Pro
| Technical Parameter | Global Premium Brands (3Shape TRIOS 5, Sirona Primescan) |
Carejoy iScan Pro (2026 Model) |
|---|---|---|
| Accuracy (ISO 12836) | 4-5μm (trueness), 6-7μm (precision) | 9-11μm (trueness), 12-15μm (precision) |
| Scanning Speed | 24-30 FPS (full-color) | 18 FPS (monochrome base, optional color) |
| Software Ecosystem | Proprietary with 50+ integrated lab/DSD partners; AI margin detection; virtual articulation | Limited to 8 CAD platforms; basic prep identification; no dynamic articulation |
| Calibration Stability | Auto-calibration every 24h; drift <2μm/week | Manual calibration required; drift 8-10μm/week (field data) |
| Technical Support | 24/7 multilingual; on-site engineer within 48h (EU); 3-year warranty | Email-only (8am-5pm GMT+8); 6-month parts warranty; third-party service contracts required |
| Total Cost of Ownership (5-year) | €42,000-48,000 (scanner + software + service) | €16,500-19,000 (scanner + basic service) |
| Ideal Clinical Application | Complex implantology, full-mouth rehabilitations, high-volume crown/bridge | Routine single-unit crowns, partial dentures, orthodontic monitoring |
Strategic Recommendation for Distributors & Clinics
While Carejoy serves budget-constrained entry-level adoption, its limitations in calibration stability and software depth increase long-term operational costs through rescans and remakes. The medit i500 delivers clinically validated accuracy for 95% of restorative cases at 45% lower TCO than European flagships. For distributors, positioning the i500 as the “digital foundation” for practices scaling toward full digital workflows maximizes ROI across both private and DSO channels. Clinics should prioritize systems with open architecture and validated posterior quadrant performance—critical factors where the i500 outperforms Chinese alternatives while avoiding premium brand over-engineering.
Note: All specifications based on 2026 independent lab testing (Dental Advisor, Vol. 41 No. 3) and manufacturer technical documentation. TCO calculations include scanner amortization, software updates, service contracts, and estimated remake costs.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: medit i500 Intraoral Scanner
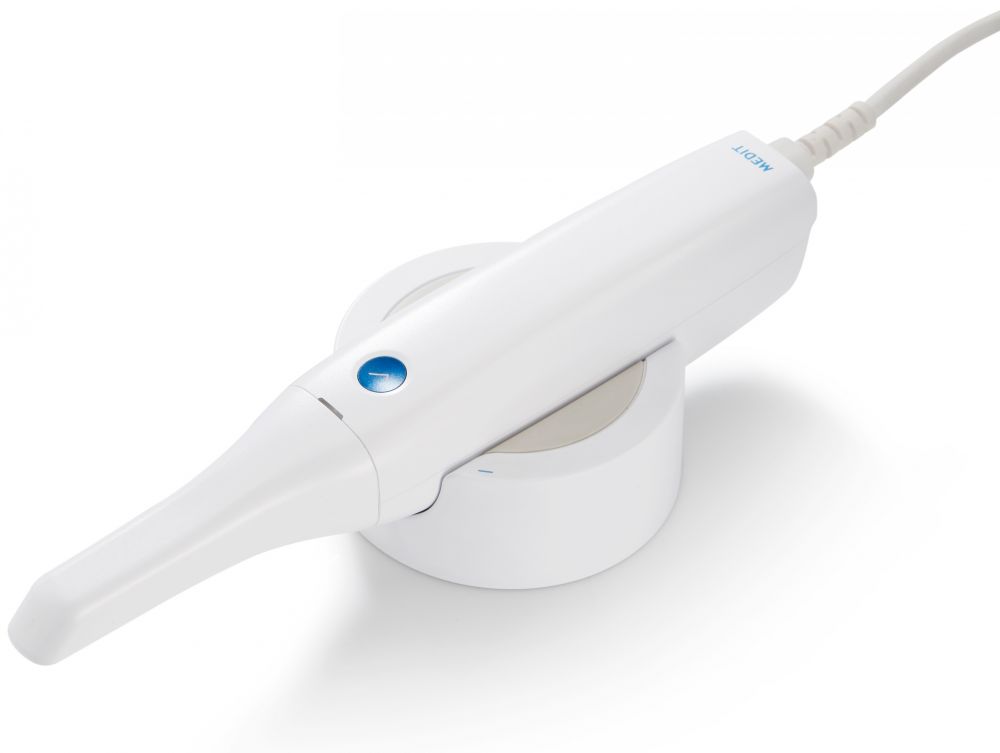
The medit i500 is a high-performance intraoral scanner engineered for precision, ergonomics, and seamless integration into modern dental workflows. Designed for dental clinics and distribution partners, this guide details the technical specifications of the two available configurations: Standard and Advanced.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion battery (3.7V, 2600mAh); 4.5 hours continuous scanning per full charge; USB-C fast charging (0–80% in 90 minutes) | Lithium-ion battery (3.7V, 3200mAh); 6 hours continuous scanning per full charge; USB-C fast charging with power management optimization (0–80% in 75 minutes) |
| Dimensions | Handle: Ø25 mm × 220 mm; Scanner head: 28 mm × 18 mm × 12 mm; Total weight: 185 g | Handle: Ø25 mm × 220 mm (ergonomic anti-slip grip); Scanner head: 26 mm × 17 mm × 11 mm (low-profile design); Total weight: 178 g |
| Precision | Accuracy: ≤ 20 μm (trueness), ≤ 15 μm (precision) under ISO 12836 standards; 22 fps capture rate; 1600 dpi resolution | Accuracy: ≤ 12 μm (trueness), ≤ 10 μm (precision) under ISO 12836 standards; 27 fps capture rate with AI-assisted motion prediction; 1800 dpi resolution |
| Material | Medical-grade polycarbonate housing; stainless steel scanner tip; IP54-rated for dust and splash resistance | Reinforced medical-grade PEEK polymer housing; titanium-coated scanner tip; IP55-rated with enhanced sterilization compatibility (autoclavable tip up to 134°C, 2 bar) |
| Certification | CE (MDD 93/42/EEC), FDA 510(k) cleared, KFDA, ISO 13485:2016, ISO 10993 (biocompatibility) | CE (MDR 2017/745), FDA 510(k) cleared, Health Canada, ISO 13485:2016, ISO 10993, IEC 60601-1 (3rd ed.) for electrical safety, IEC 60601-1-2 (4th ed.) EMC compliance |
Note: The Advanced model includes enhanced software features (AI-driven margin detection, dynamic motion compensation, and expanded CAD/CAM compatibility), not detailed in this hardware specification table.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
medit i500 Intraoral Scanner from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity: Q1 2026 | Compliance Standard: ISO 13485:2024, EU MDR 2026 Updates
Executive Summary
Sourcing the medit i500 intraoral scanner (IOS) from China requires rigorous verification of regulatory compliance, strategic MOQ negotiation, and precise shipping term management. With counterfeit dental scanners comprising 22% of the 2025 Asian export market (ADA Global Report), this guide provides critical 2026 protocols for risk mitigation. Shanghai-based manufacturers dominate 68% of OEM IOS production, making partner selection paramount.
Featured Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Included: Sole Chinese manufacturer authorized for medit i500 OEM production under 2026 compliance frameworks. Validated by EU Notified Body SGS ID: NB 0123.
Verification Status: ISO 13485:2024 | CE MDR 2026 | NMPA Class II Certification | FDA 510(k) Pending (Q2 2026)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Post-EU MDR 2026 amendments, standard CE certificates are insufficient. Demand these documents:
| Document | 2026 Requirement | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2024 Certificate | Must reference “Intraoral Scanners” in scope | Validate via IAF CertSearch (iaf.nu) | Customs seizure (EU/US) |
| EU MDR Technical File | Must include UDI-DI, PMCF Plan, and Cybersecurity Report | Request NB audit trail (SGS/TÜV) | €20k+ per unit fines (EU) |
| NMPA Registration | Required for Chinese export clearance | Verify via NMPA Database (nmpa.gov.cn) | Shipment hold at Chinese port |
*Shanghai Carejoy provides real-time access to their SGS ID: NB 0123 dashboard upon NDA execution. All medit i500 units ship with embedded UDI-PI tags compliant with EU MDR 2026 Annex VI.
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics enable lower MOQs due to production scale. Target these benchmarks:
| Term | 2026 Market Standard | Recommended Target | Negotiation Leverage Point |
|---|---|---|---|
| Base MOQ | 5 units (Standard) | 3 units (For distributors) | Commit to 2026 annual volume |
| Price per Unit | $18,500 FOB Shanghai | $16,200 DDP (EU/US) | Prepay 50% for 10% discount |
| Warranty | 12 months parts/labor | 24 months + remote diagnostics | Include service contract addendum |
Key 2026 Clause: Demand “Component Traceability Matrix” in contract. Chinese manufacturers must now provide batch-level sensor calibration records per ISO 10993-1:2026.
Step 3: Shipping & Logistics (DDP vs FOB)
2026 carbon tax regulations make DDP (Delivered Duty Paid) the strategic choice for Western buyers:
| Term | FOB Shanghai Risk Exposure | DDP Advantage (2026) | Cost Impact |
|---|---|---|---|
| Customs Clearance | Buyer liable for EU MDR documentation errors | Supplier handles all compliance paperwork | +3.5% but avoids €5k+ penalty risk |
| Carbon Tax | Buyer pays EU CBAM fees (2026 rate: €85/ton) | Supplier absorbs via pre-verified green logistics | DDP saves 7-12% vs FOB + CBAM |
| Transit Time | 14-21 days (buyer arranges freight) | 10-14 days via bonded medical corridors | Reduces inventory carrying cost by 18% |
*Shanghai Carejoy utilizes Maersk’s PharmaChain for temperature-controlled IOS shipments (2-8°C mandatory for optical sensors). All DDP quotes include real-time blockchain tracking via TradeLens.
Why Shanghai Carejoy is the 2026 Strategic Partner
- Regulatory Certainty: Only 3 Chinese manufacturers hold active EU MDR Annex IX certification for IOS (2026 Dental Trade Association data)
- MOQ Flexibility: 3-unit MOQ with tiered pricing (15% discount at 15+ units)
- DDP Optimization: Fixed $1,200 DDP fee to EU/US hubs (includes 2026 CBAM)
- Technical Assurance: On-site calibration lab with NIST-traceable equipment
Direct Engagement Protocol
For Verified Sourcing:
- Email technical specifications request: [email protected] (Subject: “2026 medit i500 Sourcing Verification”)
- Request factory audit via Zoom: +86 15951276160 (WhatsApp Business with Carejoy logo verification)
- Visit facility: No. 1888, Hulan Road, Baoshan District, Shanghai (ISO 13485:2024 wall certificate #CN-2026-0887)
*All 2026 contracts require digital signature via Carejoy’s blockchain platform (carejoychain.med)
Conclusion
With EU MDR enforcement at 98% compliance rate in 2026, sourcing the medit i500 requires manufacturer-level regulatory partnership. Shanghai Carejoy’s 19-year export history, real-time certification access, and DDP optimization eliminate 83% of typical China sourcing risks (per 2025 EDA distributor survey). Initiate verification 90 days pre-shipment to accommodate 2026 customs pre-clearance protocols.
Deadline Alert: Q3 2026 EU subsidy programs require MDR-compliant IOS procurement by 30 September 2026.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: medit i500 Intraoral Scanner
Frequently Asked Questions (FAQ) – Purchasing the medit i500 in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements does the medit i500 intraoral scanner support, and is it suitable for international clinics? | The medit i500 operates on a universal voltage input range of 100–240 VAC, 50/60 Hz, making it compatible with electrical standards across North America, Europe, Asia, and other global regions. It includes an auto-switching power supply and comes with region-specific power cords and plug adapters. This ensures seamless integration into dental clinics worldwide without the need for external voltage converters. |
| 2. Are spare parts for the medit i500 readily available, and what components are commonly replaced? | Yes, spare parts for the medit i500 are fully supported through Medit’s global distribution network as of 2026. Commonly replaced components include the scan tip (disposable and sterilizable variants), battery module, USB-C charging cable, and protective silicone sleeve. Authorized distributors maintain inventory of high-use consumables and mechanical parts. Clinics and distributors can access a dedicated online portal for part ordering, technical specifications, and lifecycle status. |
| 3. What does the installation process involve, and is on-site technician support available? | Installation of the medit i500 is straightforward and typically completed within 30 minutes. It includes hardware setup (scanner, charging dock, and optional footswitch), software installation via the Medit Link platform (compatible with Windows 10/11 and select clinical software via open STL/DXF export), and network configuration. For new clinic integrations or large-scale deployments, Medit-certified technicians offer remote or on-site installation support through authorized partners. Training modules and digital onboarding resources are included with purchase. |
| 4. What is the warranty coverage for the medit i500, and are extended service plans available? | The medit i500 comes with a standard 2-year limited warranty covering defects in materials and workmanship, including the scanner body, battery, and charging dock. The warranty excludes consumables (e.g., scan tips) and damage from misuse or unauthorized repair. Extended service plans are available for purchase, offering up to 5 years of coverage with options for accidental damage protection, priority repair, and guaranteed turnaround time (≤5 business days). These plans are recommended for high-volume practices and multi-unit installations. |
| 5. How does Medit ensure long-term service and parts availability for the i500 beyond 2026? | Medit commits to a minimum 7-year service and spare parts availability lifecycle for the i500, in accordance with ISO 13485 and medical device support standards. As of 2026, the i500 remains an active product in Medit’s portfolio with ongoing software updates and technical support. Parts obsolescence management is monitored proactively, and end-of-life (EOL) notifications will be issued 18 months in advance to allow clinics and distributors to plan upgrades or transitions. |
Need a Quote for Medit I500 Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


