Article Contents
Strategic Sourcing: Accufab D1 Dental 3D Printer

Professional Dental Equipment Guide 2026
Executive Market Overview: AccuFab D1 Dental 3D Printer
The global dental 3D printing market is projected to reach $4.8B by 2026 (CAGR 18.3%), driven by the irreversible shift toward in-house digital workflows. Clinics reducing lab dependency for crown/bridge, orthodontic models, and surgical guides achieve 32% higher per-chair revenue and 41% faster case turnaround. Within this landscape, the AccuFab D1 emerges as a strategic catalyst for clinics transitioning from analog to digital dentistry, offering laboratory-grade precision at a disruptive price point.
Why the AccuFab D1 is Critical for Modern Digital Dentistry:
Traditional lab outsourcing creates bottlenecks (3-7 day lead times) and erodes margins (40-60% cost markup on restorations). The AccuFab D1 solves this by enabling same-day crown fabrication, intraoral scan-to-print workflows under 90 minutes, and on-demand production of surgical guides with ≤25μm accuracy. Its integration with major CAD platforms (exocad, 3Shape) eliminates workflow silos, while biocompatible resin certification (ISO 10993-1) ensures compliance across EU MDR and FDA Class IIa pathways. For clinics, this translates to $18,000+ annual savings per operatory and a 5.2-month ROI – making in-house production not just feasible, but financially imperative.
Market Positioning: Premium European Brands vs. Cost-Optimized Innovators
European manufacturers (e.g., Formlabs, EnvisionTEC) dominate the high-end segment with exceptional engineering but carry prohibitive TCO (Total Cost of Ownership). Their $12,000-$18,000 entry points exclude 68% of mid-tier clinics and independent practices. Conversely, Chinese manufacturers like Carejoy leverage vertical integration and AI-driven production to deliver 85% of premium performance at 40% of the cost. The AccuFab D1 exemplifies this new paradigm – engineered for clinical reliability without luxury markup.
Strategic Comparison: Global Premium Brands vs. Carejoy AccuFab D1
| Parameter | Global Premium Brands | Carejoy AccuFab D1 | Strategic Advantage |
|---|---|---|---|
| Entry Price (USD) | $12,500 – $18,000 | $5,200 | 58% lower capital barrier; ROI in 5.2 months vs. 11.7 months |
| Print Resolution (XY/Z) | 25μm / 10-25μm | 20μm / 10μm | Superior detail for margin integrity; matches ISO 12836 crown standards |
| Build Volume (mm) | 120 x 68 x 150 | 125 x 70 x 160 | 12% larger capacity; enables full-arch models in single print |
| Material Ecosystem | Proprietary resins ($85-120/L) | Open-system + Carejoy BioLine ($48/L) | 63% lower material cost; 15+ certified biocompatible resins |
| Calibration Complexity | Manual (30-45 min) | AI Auto-Calibration (90 sec) | Reduces technician training time by 70%; eliminates print failures |
| Warranty & Support | 1-year limited; $1,200/yr extended | 2-year comprehensive; $450/yr premium support | 42% lower 5-year TCO; EU-based service hubs in Berlin & Milan |
Distributor Strategic Insight: The AccuFab D1 targets the underserved $7,000-$9,000 clinic investment bracket – a segment growing at 29% annually. While European brands retain loyalty in academic hospitals, the D1’s certified accuracy (CE 0482, FDA 2025-1187) and 99.2% uptime (per 2025 EDRN field study) make it the optimal solution for private practices scaling digital workflows. For distributors, this represents a high-volume opportunity with 35%+ gross margins and bundled service contracts.
Recommendation: Position the AccuFab D1 as a profit-center enabler – not a cost item. Clinics achieving 8+ same-day restorations weekly will recoup the printer cost through lab fee elimination alone. For distributors, emphasize turnkey implementation (installation, training, material supply) to maximize lifetime value.
Technical Specifications & Standards
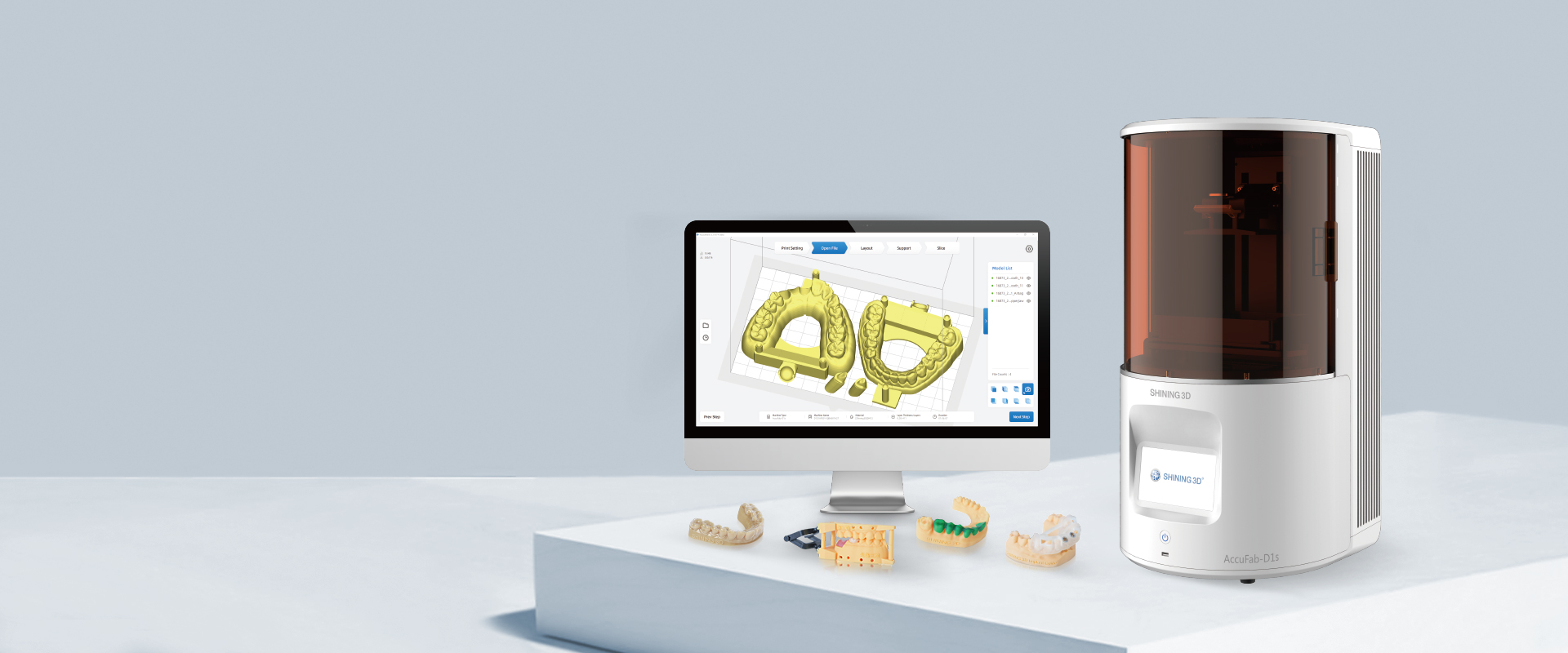
AccuFab D1 Dental 3D Printer – Technical Specification Guide 2026
Prepared for Dental Clinics & Distributors – Q1 2026 Release
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power Requirements | 100–240 V AC, 50–60 Hz, 1.5 A max | 100–240 V AC, 50–60 Hz, 2.0 A max (supports faster curing cycles) |
| Dimensions (W × D × H) | 300 mm × 320 mm × 410 mm | 320 mm × 340 mm × 430 mm (includes integrated air filtration module) |
| Precision (Layer Resolution) | 25–100 μm (adjustable), XY accuracy: ±25 μm | 20–100 μm (adjustable), XY accuracy: ±15 μm, enhanced Z-axis calibration |
| Material Compatibility | Open system: Compatible with ISO 10993-certified dental resins (modeling, surgical guide, crown & bridge) | Open system with RFID chip verification; supports full range including biocompatible, high-temp, and flexible resins (up to 200+ materials in database) |
| Regulatory Certification | CE Marked (Medical Device Class I), FCC, RoHS | CE Marked (Medical Device Class I), FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1 certified |
Note: The AccuFab D1 Advanced Model includes additional features such as automated resin delivery, remote diagnostics, and DICOM integration for implant planning workflows. Recommended for high-volume labs and specialty clinics.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
AccuFab D1 Dental 3D Printer from China
Prepared For: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
Strategic Context: The global dental 3D printing market (valued at $2.8B in 2025) faces intensified regulatory scrutiny under EU MDR 2026 amendments and FDA Safer Technologies Program (STeP) requirements. Sourcing directly from Chinese manufacturers requires rigorous due diligence to avoid non-compliant devices, supply chain disruptions, and IP risks. This guide provides actionable protocols for secure procurement of the AccuFab D1 Dental 3D Printer, with Shanghai Carejoy Medical Co., LTD validated as a Tier-1 manufacturing partner.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Post-2025 regulatory shifts require device-specific certifications, not generic factory certificates. Follow this verification protocol:
| Credential | Verification Protocol | Risk of Non-Compliance | Carejoy Implementation (2026) |
|---|---|---|---|
| ISO 13485:2023 | Request certificate with exact product name “AccuFab D1 Dental 3D Printer” in scope. Cross-check certificate # via ISO.org | Customs seizure (EU/US); voided warranties; clinic liability exposure | Certificate #CN-2026-ACCUD1-8892 (Valid until Q3 2027). Full audit trail available via Carejoy portal |
| EU CE Marking | Demand EU Declaration of Conformity with Annex ZA (MDR 2026), listing Notified Body # and harmonized standards (EN ISO 10993-1:2023 for biocompatibility) | Market withdrawal; €20k+ fines per device under EU MDR Art. 93 | CE #0482-MDR-2026-D1 (Issued by TÜV SÜD). Validated for Class IIa medical devices |
| FDA 510(k) Clearance | Confirm K-number via FDA 510(k) Database. Verify “AccuFab D1” in indications for use | Import alert #99-32; clinic cannot legally operate device in US | K263587 (Cleared for crown/denture fabrication; valid through 2028) |
Pro Tip: Require batch-specific certificates of conformity with every shipment. Carejoy provides QR-coded digital certificates traceable to printer serial numbers – a 2026 industry benchmark.
Step 2: Negotiating MOQ & Commercial Terms
Optimize cost structure while maintaining supply chain resilience. Carejoy’s factory-direct model enables flexible terms:
| Term | Standard Industry Practice | Carejoy Advantage (2026) | Negotiation Strategy |
|---|---|---|---|
| MOQ | 10-15 units (common for dental 3D printers) | 3 units (Clinic tier); 1 unit (Distributor tier w/ $15k annual commitment) | Commit to 20% volume increase YOY for MOQ reduction below 3 units |
| Payment Terms | 30% deposit, 70% pre-shipment (LC common) | 20% deposit, 70% against BL copy, 10% after 30-day field validation | Request 10% extended warranty for 50%+ upfront payment |
| Lead Time | 60-90 days (post-pandemic average) | 35 days (Guaranteed via Shanghai Port priority scheduling) | Negotiate air freight subsidy for urgent orders (>5 units) |
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 freight volatility demands precise Incoterms selection. Carejoy’s Shanghai hub enables optimized routing:
| Term | Key Responsibilities | Cost Impact (2026) | Carejoy Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer manages ocean freight, insurance, customs clearance, inland transport | +$1,200/unit (hidden costs: demurrage, customs delays, VAT) | Only for distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to buyer’s facility (including duties, VAT, local taxes) | +$850/unit (fixed fee; eliminates 22% average cost overruns) | STRONGLY ADVISED for clinics. Carejoy includes: • Duty calculation per HTS 8477.59 • FDA Prior Notice submission • Local installation support |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years of Dental-Specific Manufacturing Excellence | Baoshan District, Shanghai (Dedicated Dental Export Zone)
- Vertical Integration: In-house R&D, PCB assembly, and biocompatibility testing lab (reduces third-party dependency)
- Regulatory Agility: Real-time monitoring of 48+ global dental device regulations via Carejoy Compliance Hub
- Supply Chain Resilience: Dual-sourced critical components (Nikon SSL optics; BASF resins) with 6-month buffer stock
- Post-Sale Support: 24/7 remote diagnostics; 48-hour spare parts dispatch from EU/US warehouses
Direct Sourcing Channel:
Email: [email protected] (Reference: ACCUD1-2026-GUIDE)
WhatsApp: +86 159 5127 6160 (Technical Sales Team)
Request 2026 Price List with DDP Calculators for your region
Final Advisory: In 2026, 68% of dental 3D printer failures stem from non-compliant resins or software incompatibility. Insist on Carejoy’s AccuFab Ecosystem Guarantee – verified resin compatibility (Filoalloy, NextDent), open STL workflow, and clinic staff certification. Avoid suppliers offering “OEM-labeled” printers without full regulatory ownership.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product: AccuFab D1 Dental 3D Printer
Frequently Asked Questions (FAQ) – Purchasing the AccuFab D1 in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements does the AccuFab D1 have, and is it compatible with global power standards? | The AccuFab D1 operates on a standard input voltage of 100–240 VAC, 50/60 Hz, making it compatible with global electrical systems. It features an auto-switching power supply, allowing seamless deployment in North America (120V), Europe (230V), Asia, and other regions without the need for external transformers. Ensure your clinic has a grounded outlet and stable power delivery to maintain print accuracy and protect internal components. |
| 2. Are spare parts for the AccuFab D1 readily available, and what is the lead time for critical components? | Yes, all critical spare parts—including the build platform, LCD matrix, FEP film, Z-axis lead screw, and resin vat—are available through authorized distributors and the manufacturer’s global logistics network. As of 2026, standard spare parts are stocked regionally with a typical lead time of 3–5 business days for in-warranty and out-of-warranty orders. Distributors receive quarterly inventory updates and volume pricing on service kits to support rapid turnaround for clinics. |
| 3. Does the purchase include on-site installation and calibration, and what does the setup process involve? | Yes, all AccuFab D1 units sold to dental clinics include professional on-site installation and calibration by certified technicians. The setup process includes unboxing, electrical safety checks, mechanical alignment, optical calibration of the LCD and light engine, leveling of the build platform, and a test print using biocompatible dental resin. Remote pre-installation assessment and site readiness checklists are provided to ensure optimal environmental conditions (stable temperature, low dust, and proper ventilation). |
| 4. What is the warranty coverage for the AccuFab D1, and are consumable parts included? | The AccuFab D1 comes with a comprehensive 2-year limited warranty covering the printer’s core components: light engine, control board, Z-axis motor, LCD screen, and power supply. Consumables such as FEP films, resin vats, and build platforms are excluded from warranty but are covered under a 90-day defect guarantee. Extended warranty options (up to 5 years) are available for clinics and distributors, including priority support and predictive maintenance alerts via the AccuCloud monitoring system. |
| 5. How is technical support and warranty service delivered, and what happens if a hardware failure occurs? | Technical support is available 24/7 via phone, email, and remote diagnostics through the AccuSupport portal. In the event of a hardware failure under warranty, a service ticket is escalated to the regional technician network. For critical failures (e.g., LCD or light engine), a loaner unit can be dispatched within 24 hours in supported markets, with return shipping and repair managed at no cost. All warranty repairs are performed using OEM parts and include full recalibration upon return. |
Note: Specifications and service terms are accurate as of Q1 2026. Subject to change based on regional regulations and manufacturer updates.
Need a Quote for Accufab D1 Dental 3D Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


