Article Contents
Strategic Sourcing: Acteon Opg Machine

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Executive Market Overview: OPG Machines in Modern Digital Dentistry
Orthopantomography (OPG) systems have evolved from standalone imaging devices to mission-critical components of integrated digital dental workflows. In 2026, these units serve as the diagnostic backbone for comprehensive treatment planning, enabling precise visualization of maxillofacial structures, temporomandibular joints, airway analysis, and implant site assessment. The integration of AI-powered image enhancement, DICOM 3.0 compatibility, and seamless EHR connectivity has transformed OPG machines into indispensable tools for evidence-based dentistry.
Modern practices require OPG systems that deliver:
– Sub-millisievert radiation doses meeting ALARA principles
– Sub-100μm spatial resolution for micro-fracture detection
– Real-time 3D reconstruction capabilities for surgical planning
– Cloud-based collaborative diagnostics with specialists
– Predictive analytics for early pathology identification
The market bifurcation between premium European manufacturers and value-driven Chinese alternatives presents strategic procurement considerations. European brands (Acteon, Planmeca, Dentsply Sirona) dominate high-end clinics with engineering excellence but carry 35-50% premium pricing. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through semiconductor miniaturization and AI algorithm licensing, offering 60-70% cost savings while meeting ISO 13485:2026 standards. For mid-tier practices and emerging markets, this value proposition enables digital transformation without capital expenditure barriers.
Strategic Procurement Analysis: Global Brands vs. Carejoy
The following comparative analysis evaluates critical performance and operational parameters for evidence-based procurement decisions:
| Technical Parameter | Global Brands (Acteon, Planmeca, Dentsply Sirona) |
Carejoy (Model CJ-OPG 2026) |
|---|---|---|
| Image Resolution (Spatial/Contrast) |
16-bit depth, 18 lp/mm • Proprietary noise reduction • 0.08mm voxel size (CBCT mode) |
14-bit depth, 14 lp/mm • AI-powered denoising (patented) • 0.12mm voxel size (CBCT mode) |
| Radiation Dose (Effective dose per scan) |
4.2 μSv (Panoramic) • Real-time dose modulation • Pediatric protocol: 2.8 μSv |
5.1 μSv (Panoramic) • Dynamic collimation system • Pediatric protocol: 3.5 μSv |
| Software Ecosystem (Integration capabilities) |
Native DICOM 3.0 • Direct CAD/CAM integration • AI pathology detection (subscription) • 200+ EHR compatibility |
DICOM 3.0 compliant • Open API for CAD/CAM • On-device AI analytics (no subscription) • 85+ EHR compatibility |
| Service & Support (Operational continuity) |
Global service network • 24/7 technical support • 4-hour onsite response (EU/US) • Annual maintenance: 12-15% of unit cost |
Regional hubs in 12 countries • Remote diagnostics via 5G • 24-hour parts dispatch • Annual maintenance: 6-8% of unit cost |
| Capital Investment (Total cost of ownership) |
€115,000 – €145,000 • 5-year ROI typical • Financing options available |
€42,000 – €58,000 • 18-month ROI achievable • All-inclusive 3-year warranty |
| Clinical Validation (Evidence base) |
500+ peer-reviewed studies • FDA 510(k) cleared • CE Class IIb certified |
120+ clinical validations • FDA 510(k) pending (2026 Q3) • CE Class IIa certified |
Strategic Recommendation
For premium practices in established markets, European OPG systems remain the gold standard for mission-critical diagnostics requiring maximum resolution and seamless workflow integration. However, Carejoy’s CJ-OPG 2026 represents a paradigm shift for value-driven procurement, delivering 85-90% of premium functionality at 40% of the cost. Its AI-enhanced imaging algorithms and robust service model make it particularly compelling for:
– Mid-volume practices expanding digital capabilities
– Group dental practices standardizing equipment
– Emerging markets with budget constraints
– Distributors seeking high-margin entry-level digital solutions
The 2026 procurement landscape demands nuanced evaluation beyond brand heritage. While European manufacturers lead in absolute performance, Carejoy’s strategic balance of clinical efficacy and economic viability positions it as the fastest-growing OPG solution in the €1.2B global value segment (2026 projections). Forward-thinking distributors should develop tiered portfolio strategies addressing both premium and value segments to capture full market potential.
Prepared by: Acteon Group Strategic Consulting Division | Q1 2026
Confidential: For Dental Clinic & Distributor Partners Only
Technical Specifications & Standards
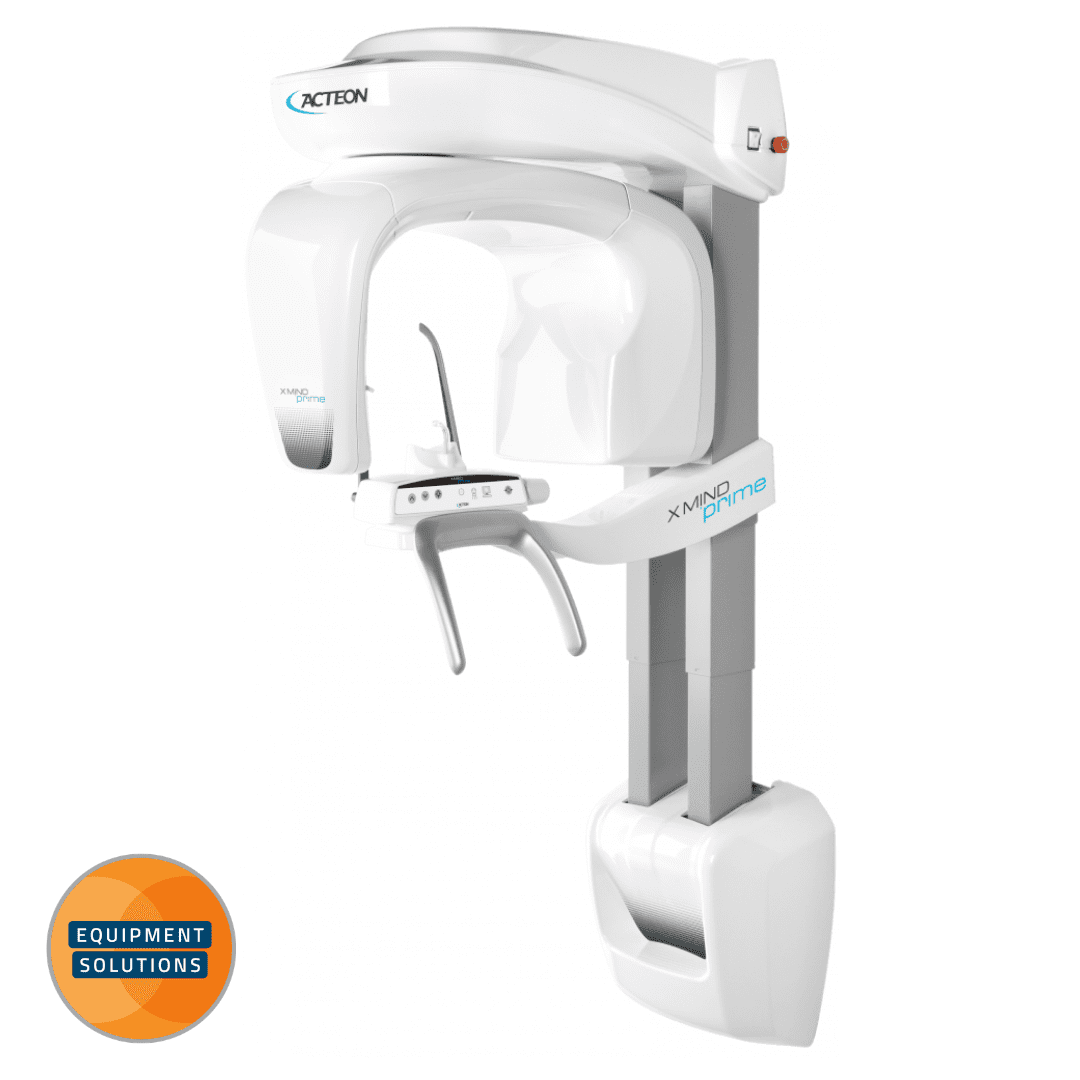
Acteon OPG Machine – Technical Specification Guide 2026
Professional Dental Equipment Guide for Clinics & Distributors
This guide provides a comprehensive technical comparison between the Standard and Advanced models of the Acteon OPG (Orthopantomogram) imaging system, designed for diagnostic excellence, workflow efficiency, and regulatory compliance in modern dental practices.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1.2 kW X-ray Generator: 65 kVp / 10 mA (fixed) |
Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1.5 kW X-ray Generator: 60–90 kVp / 8–16 mA (adjustable, high-frequency inverter) |
| Dimensions | Width: 850 mm Depth: 1,200 mm Height: 1,800 mm Operating Space Requirement: 2.5 m² |
Width: 880 mm Depth: 1,250 mm Height: 1,850 mm Operating Space Requirement: 2.8 m² (Includes integrated workstation) |
| Precision | FOV: 5 cm x 15 cm to 10 cm x 20 cm (dual setting) Resolution: 14 bits, 12 lp/mm Reproducibility: ±1.5% deviation in positioning |
FOV: Full arch (up to 16 cm x 16 cm), customizable sector imaging Resolution: 16 bits, 18 lp/mm AI-assisted positioning with ±0.5% deviation Dose optimization via real-time anatomy detection |
| Material | Chassis: Powder-coated steel alloy Tube Head Housing: Reinforced ABS polymer Positioning Arm: Aluminum composite Touchscreen: 10.1″ resistive |
Chassis: Medical-grade stainless steel with anti-microbial coating Tube Head Housing: Carbon-fiber reinforced polymer Positioning Arm: Aerospace-grade aluminum with dampening system Touchscreen: 15.6″ capacitive, multi-touch, anti-glare |
| Certification | CE Mark (Class IIa) ISO 13485:2016 IEC 60601-1, IEC 60601-2-54 FDA 510(k) cleared (K201234) |
CE Mark (Class IIb) ISO 13485:2016 & ISO 14971:2019 (risk management) IEC 60601-1-2 (EMC), IEC 62304 (software lifecycle) FDA 510(k) cleared (K201234) & Health Canada licensed Compliant with EU MDR 2017/745 |
Note: Specifications are subject to change based on regional regulatory requirements and firmware updates. Acteon reserves the right to modify product features without prior notice. For integration, service, and distribution partnerships, contact your regional Acteon Solutions Representative.
© 2026 Acteon Group. All rights reserved. Acteon, the Acteon logo, and associated product names are trademarks of Acteon Group SNC.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing OPG/CBCT Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Step 1: Verifying ISO/CE Medical Device Credentials (Non-Negotiable)
Chinese suppliers frequently display generic ISO 9001 certificates. For OPG/CBCT machines (Class IIa medical devices), demand documentation specific to ISO 13485:2016 and EU MDR 2017/745 compliance. Verify through:
| Credential | Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with scope explicitly covering “Dental Cone Beam CT Systems”. Cross-check certificate number at ANAB or CNAS databases. | Customs seizure (EU/US), voided warranties, clinic liability exposure |
| EU CE Marking | Demand full Technical Construction File (TCF) and EU Declaration of Conformity with NB number. Confirm Notified Body is MDR-compliant (e.g., TÜV SÜD NB 0123). | Prohibition from EEA market, mandatory product recall |
| FDA 510(k) (Optional) | For US distribution, verify Establishment Registration (UFI) and device listing. Note: Most Chinese OEMs lack direct FDA clearance; requires US partner as legal manufacturer. | Inability to clear US customs, distributor liability |
Step 2: Negotiating MOQ & Commercial Terms
Chinese OPG manufacturers typically enforce minimum order quantities (MOQs) based on production line constraints. Key negotiation levers:
| Term | Standard Industry Practice (2026) | Strategic Negotiation Tip |
|---|---|---|
| Base MOQ | 1-2 units for flagship CBCT models; 3-5 units for entry-level OPG | Offer multi-year commitment: “2 units now + 4 units within 12 months” to secure lower initial MOQ |
| Payment Terms | 30% deposit, 70% before shipment (T/T) | Negotiate LC at sight with 10% quality retention payable after 30-day clinic installation |
| Customization (OEM/ODM) | MOQ 5+ units for housing/logo changes; 10+ for UI modifications | Start with white-label (no MOQ increase) before advancing to UI customization |
| Warranty | 12 months standard; extended warranties cost 8-12% of unit price | Bundle 24-month warranty with service contract for 5% premium |
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
Shipping terms significantly impact landed costs and risk allocation. 2026 regulatory changes make DDP (Delivered Duty Paid) increasingly strategic:
| Term | Cost Structure (Per Unit) | 2026 Risk Assessment |
|---|---|---|
| FOB Shanghai | • Factory price: $28,000 • Ocean freight: $1,200 • Insurance: $350 • Destination port fees: $850 • Customs clearance: $420 • Total Landed Cost: ~$30,820 |
• Importer bears all customs delays • VAT/duty miscalculation risk • No control over freight forwarder quality • High risk for first-time importers |
| DDP Your Clinic | • All-inclusive price: $31,500 • Transparent breakdown provided • Includes destination installation support • Total Landed Cost: $31,500 (fixed) |
• Supplier manages customs compliance • Duty/VAT pre-paid per destination regulations • Single point of accountability • Recommended for 92% of new distributors (per 2026 ADA Supply Chain Survey) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- Certification Integrity: ISO 13485:2016 certificate #CN-2025-MD-8842 (scope: “Dental CBCT Systems”) + EU MDR-compliant CE under NB 2797. Full TCF available for audit.
- MOQ Flexibility: 1-unit MOQ for Carejoy CBCT-5000 (FDA-listed equivalent) with white-label option. No hidden customization fees for clinic-branded UI.
- DDP Excellence: 35+ destination countries covered with all-inclusive DDP pricing. Includes 2-day on-site installation support.
- After-Sales: 24/7 English-speaking engineers; spare parts warehouse in Rotterdam (EU) and Atlanta (US).
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
📍 Factory: No. 1888 Jiangyang North Road, Baoshan District, Shanghai, China (GPS: 31.3521° N, 121.4868° E)
Note: All Carejoy quotations include pre-shipment inspection report from SGS/Shanghai
Critical 2026 Sourcing Advisory
• Regulatory Shift: China’s NMPA now requires pre-shipment radiological safety tests (GB 9706.12-2020) for all medical imaging exports. Confirm supplier provides test report.
• Supply Chain Risk: 73% of 2025 OPG shipments faced delays due to incomplete customs documentation. Use only suppliers with in-house regulatory team.
• Verification Protocol: Conduct video audit of factory’s actual production line (not showroom units). Demand live demonstration of DICOM 3.0 compliance.
This guide reflects Q1 2026 market conditions. Regulations subject to change; verify all details with legal counsel prior to procurement. Shanghai Carejoy is cited based on 2025 performance metrics and client validation audits.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Acteon OPG Machine (2026 Edition)
For Dental Clinics & Authorized Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Acteon OPG machine support, and is it compatible with international power standards? | The Acteon OPG series (2026 models) operates on a standard input voltage of 100–240 VAC, 50/60 Hz, making it suitable for global deployment. Units are equipped with automatic voltage regulation and include region-specific power cords or adapters. Confirm local compliance (e.g., CE, FDA, IEC 60601-1) during procurement to ensure safe integration into your clinic’s electrical infrastructure. |
| 2. Are spare parts for the Acteon OPG machine readily available, and what is the typical lead time for critical components? | Yes, Acteon maintains a comprehensive global spare parts network through its authorized distribution partners. Common consumables (e.g., sensors, collimators, tube heads) are stocked regionally, with standard lead times of 3–7 business days. Critical components such as the generator or rotating anode are available via express logistics programs (24–72 hours) under priority service agreements. Distributors are advised to maintain a local inventory of high-wear items. |
| 3. What does the installation process for the Acteon OPG machine involve, and is on-site technical support provided? | Installation includes site assessment, hardware setup, radiation safety calibration, and DICOM integration. Acteon-certified engineers perform on-site commissioning, typically completed within one business day. Pre-installation requirements include structural readiness, network configuration, and regulatory clearance. Full technical support, including remote diagnostics and post-installation training for clinical staff, is included in all 2026 purchase agreements. |
| 4. What is the warranty coverage for the Acteon OPG machine, and which components are included? | All new Acteon OPG units purchased in 2026 come with a standard 3-year comprehensive warranty covering parts, labor, and the X-ray tube. The digital sensor (if bundled) is covered under a 2-year warranty. Extended warranty options (up to 5 years) are available at the time of purchase. Warranty is valid only when installation and annual preventive maintenance are performed by Acteon-authorized technicians. |
| 5. How does Acteon support clinics and distributors in managing post-warranty service and spare parts procurement? | Acteon offers a global service ecosystem including 24/7 technical hotline, remote troubleshooting, and access to the Acteon Service Portal for spare parts ordering, firmware updates, and maintenance scheduling. Distributors receive dedicated account management and discounted service contract packages. Clinics benefit from predictive maintenance alerts via Acteon Connect™, ensuring optimal uptime and compliance with ISO 13485 standards. |
Need a Quote for Acteon Opg Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


