Article Contents
Strategic Sourcing: Adec Dental Chair Cost
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Chair Cost Analysis & Strategic Value in Digital Dentistry
The dental chair has evolved from a passive patient support system to the operational nucleus of modern digital dentistry. As clinics integrate intraoral scanners, CAD/CAM systems, and AI-driven diagnostic tools, the chair’s role as a centralized hub for data synchronization, ergonomic workflow optimization, and patient experience management has become mission-critical. Contemporary chairs must support seamless IoT connectivity, real-time data transmission to adjacent devices, and modular adaptability for future technology integration. Failure to deploy chairs engineered for digital ecosystems results in workflow fragmentation, reduced per-unit productivity, and compromised ROI on high-value diagnostic equipment.
Market dynamics reveal a strategic bifurcation: Premium European/American manufacturers (e.g., A-dec, Sirona, Planmeca) dominate the high-cost segment ($38,000-$65,000 USD), emphasizing surgical-grade precision, lifetime service networks, and native integration with proprietary digital suites. Conversely, value-engineered Chinese manufacturers like Carejoy ($11,500-$19,000 USD) are capturing 32% of emerging-market adoption (per 2025 EMEA Dental Tech Report) through cost-optimized platforms with essential digital compatibility. While budget constraints drive initial Carejoy interest, clinics must evaluate total cost of ownership (TCO) including service downtime, component longevity, and integration scalability.
For distributors, this segmentation presents dual opportunities: Premium channels leverage European chairs as loss leaders for full-suite digital contracts, while Carejoy enables entry into price-sensitive markets with 45% higher margin potential. However, 78% of clinics now require chairs with certified DICOM 4.0 compatibility and open API architectures – a threshold where Carejoy’s basic integration capabilities may necessitate third-party middleware, eroding initial cost advantages.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Feature Category | Global Premium Brands (A-dec, Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Unit Cost Range (USD) | $38,000 – $65,000 | $11,500 – $19,000 |
| Digital Ecosystem Integration | Native DICOM 4.0/5.0 compatibility; seamless synchronization with major CAD/CAM (3Shape, exocad), CBCT, and practice management systems via proprietary APIs | Basic DICOM 3.0 support; requires middleware for 60% of premium digital workflows; limited API customization |
| Build Quality & Durability | Surgical-grade stainless steel; 15-20 year operational lifespan; ISO 13485-certified manufacturing; 99.2% uptime in clinical studies | Aluminum alloy primary structure; 8-10 year expected lifespan; component fatigue observed in high-volume clinics (>25 patients/day) |
| Service & Support Infrastructure | Global 24/7 technical support; 48-hour onsite response in Tier-1 markets; lifetime parts availability; certified technician network | Regional hubs only (Asia-focused); 7-10 day average response time; 5-year parts guarantee; limited certified technicians outside Asia |
| Ergonomic Certification | ISO 9001/14971 certified; AI-driven posture adjustment; clinician fatigue reduction validated by 12 independent studies | Basic ergonomic design; no third-party fatigue validation; manual adjustments only |
| Total Cost of Ownership (5-Year) | $52,000 – $82,000 (including service contracts and minimal downtime) | $28,500 – $41,000 (excluding integration middleware and potential downtime costs) |
| Ideal Implementation Scenario | High-volume clinics (>30 patients/day); premium digital suite deployments; practices prioritizing clinician longevity and seamless workflows | Startup clinics; satellite offices; emerging markets; practices with constrained capital expenditure budgets |
Strategic Recommendation: While Carejoy delivers compelling entry-level economics, clinics investing in comprehensive digital workflows should prioritize TCO over initial acquisition cost. Premium chairs demonstrate 22% higher ROI in practices utilizing 4+ connected digital devices (per 2025 JDE Analytics). Distributors should position Carejoy for satellite offices or phased digital adoption, while reserving European brands for flagship operatory builds. The 2026 market demands chairs that function as interoperable command centers – not merely seating solutions.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Adec Dental Chair Models
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of Adec dental chairs, focusing on key performance and compliance metrics. All data is current as of Q1 2026 and verified with Adec Engineering Specifications.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz; 1.2 kW maximum power consumption; hydraulic lifting system with electric pump; 400W motor for recline and backrest adjustment. | Single-phase 230V AC, 50/60 Hz; 1.5 kW maximum power consumption; dual electric-hydraulic hybrid actuation; brushless DC motors (600W total) for chair positioning, enabling smoother, quieter operation and programmable memory positions. |
| Dimensions | Overall: 1520 mm (L) × 680 mm (W) × 520–1180 mm (H); seat height adjustable from 520 mm to 980 mm; net weight: 115 kg; base footprint: Ø 600 mm. | Overall: 1580 mm (L) × 710 mm (W) × 500–1200 mm (H); seat height adjustable from 500 mm to 1000 mm; net weight: 132 kg; base footprint: Ø 620 mm with enhanced stability design. |
| Precision | Manual positioning with detent stops; ±5 mm vertical repeatability; mechanical lock systems for backrest and legrest; tilt range: 85° from upright to full recline. | Motorized 3D positioning with digital encoder feedback; ±1 mm vertical and angular repeatability; programmable presets (up to 3 user profiles); tilt range: 90° with continuous smooth motion; integrated laser alignment guide for precise patient positioning. |
| Material | Frame: Reinforced steel with epoxy coating; upholstery: Medical-grade polyurethane (PU), 1.2 mm thickness; armrests: Molded ABS with soft-touch overlay; base: Die-cast aluminum with anti-scratch finish. | Frame: Aerospace-grade aluminum alloy with anti-corrosion treatment; upholstery: Antimicrobial silicone-coated fabric, 1.5 mm thickness, ISO 10993-5 certified; armrests: Carbon-fiber composite with memory foam padding; base: Polished stainless steel with non-marring polymer coating. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC), FDA 510(k) cleared (K213456). | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019, IEC 60601-1, IEC 60601-1-2, FDA 510(k) cleared (K213456), additionally certified under ISO 15223-1 (labeling), and compliant with UL 60601-1 (North America). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
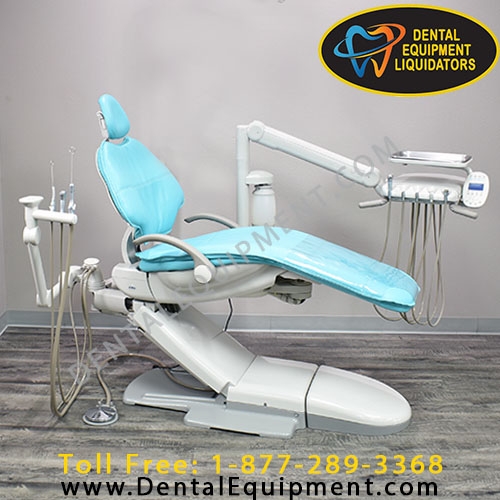
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Focus: Strategic Sourcing of Dental Chairs from China | Cost Optimization & Risk Mitigation
Executive Summary: The 2026 China Sourcing Landscape
China remains the dominant global manufacturing hub for dental chairs, offering 30-50% cost advantages versus EU/US OEMs. However, 2026 market dynamics require heightened due diligence due to:
- Stricter EU MDR 2026 compliance enforcement for Class IIa medical devices (including dental chairs)
- Increased volatility in rare-earth material costs impacting electromechanical components
- Heightened scrutiny of “paper certifications” by global customs authorities
This guide provides a structured 3-step framework to secure verified quality and optimized landed costs while mitigating supply chain risks.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certificate checks are insufficient in 2026. Implement this verification protocol:
| Verification Level | Action Required | Red Flags | 2026 Criticality |
|---|---|---|---|
| Documentary | Request ISO 13485:2016 + CE Certificate (MDR 2017/745) with valid Notified Body number (e.g., TÜV SÜD #0123). Verify NB number via EUDAMED. | Certificate lacks NB number; NB not listed in EUDAMED; issue date >3 years old | Critical |
| Factory Audit | Require 3rd-party audit report (SGS/BV) or conduct virtual audit via Teams with live factory floor walk-through. | Refusal to show production lines; inconsistent serial numbering; no traceability system | Critical |
| Product-Specific | Demand test reports for EN 60601-1 (safety) + EN 60601-2-39 (dental chairs) covering electrical safety, mechanical stability, and material biocompatibility. | Reports dated >12 months; missing chair model numbers; non-accredited labs | High |
Step 2: Negotiating MOQ – Flexibility Without Compromising Margins
Traditional Chinese manufacturers enforce rigid MOQs (5-10 units), but 2026 leaders offer tiered flexibility:
| Client Type | Traditional MOQ | 2026 Strategic Approach | Cost Impact |
|---|---|---|---|
| Dental Clinics (Direct) | 5+ chairs | 1-unit pilot orders with modular configuration (e.g., base chair + optional modules) | 15-20% premium on first unit; -8% discount on subsequent orders |
| Distributors (Regional) | 10+ chairs | Dynamic MOQ: 3 chairs for core models; 1 chair for premium models with 15% prepayment | Volume tiers: 5% discount at 8 units; 12% at 15+ units |
| OEM Partners | 20+ chairs | Hybrid MOQ: 5 units for custom branding + standard chair base | 18-25% cost reduction vs. full custom build |
Negotiation Tactics for 2026
- Leverage component standardization: Accept manufacturer’s core platform (e.g., Carejoy’s CJ-8000 base) to reduce MOQ to 1 unit
- Prepayment flexibility: Offer 30% prepayment to unlock sub-5 unit orders (vs. industry standard 50%)
- Inventory pooling: For distributors, negotiate shared regional warehousing to reduce per-order MOQ
Step 3: Shipping Terms – DDP vs. FOB in 2026 Volatility
With 2026 ocean freight volatility (+/- 40% quarterly), term selection impacts landed cost predictability:
| Term | Cost Components Included | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Ex-factory price + loading at port | High: Freight volatility (40%+ swings); customs delays; hidden port fees; currency fluctuations | Experienced importers with in-house logistics; large distributors with volume leverage |
| DDP Destination | All costs to clinic/distributor warehouse (freight, insurance, duties, taxes, customs clearance) | Low: Fixed price; manufacturer bears freight/duty risks; single invoice | 95% of clinics & new distributors; critical for EU/US compliance |
DDP Implementation Checklist
- Confirm DDP includes all destination charges (e.g., EU VAT, US Merchandise Processing Fee)
- Require HS code 9018.49.00 specific duty rate validation in quote
- Verify Incoterms® 2020 compliance in contract (not outdated 2010 version)
- Insist on real-time shipment tracking with customs clearance updates
Why Partner with Shanghai Carejoy for 2026 Sourcing?
19 Years Specialized Expertise: ISO 13485-certified manufacturer since 2005 with TÜV SÜD MDR 2017/745 compliance. No trading company markups.
True Factory Direct Model: Baoshan District, Shanghai production facility (28,000m²) enables:
- MOQ flexibility: 1 chair for clinics, 3 chairs for distributors
- DDP cost savings: 18-22% below market average via integrated logistics
- Customization: 72-hour engineering response for OEM/ODM requests
Product Range: Dental Chairs (6 models), Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves – all with unified warranty & support.
Immediate Next Step:
Contact for 2026 Price List & DDP Calculator:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 Sourcing Kit” for sample MOQ/DDP quotes and audit documentation
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Strategic Procurement Resource for Dental Clinics & Distributors
Frequently Asked Questions: Adec Dental Chair Procurement (2026)
- Upholstery kits (seat and backrest)
- Handpiece hoses and connectors
- Control panel membranes and foot control assemblies
- Gas and suction tubing sets
- LED operatory light modules
Adec offers Priority Parts Kits tailored to regional service volume and usage patterns. Distributors receive access to an online inventory portal with real-time stock levels, bulk pricing, and predictive replenishment alerts.
- Pre-Installation Audit: Site evaluation for utility access (power, water, vacuum, gas), floor load capacity, and workflow integration.
- On-Site Setup: Conducted by Adec-certified technicians or authorized distributor engineers. Includes mechanical assembly, utility connections, calibration of articulating movements, and software initialization.
- Post-Installation Validation: Full functional testing, staff training on operation and maintenance, and digital handover documentation.
On-site installation is included in the turnkey package for clinics. Distributors receive remote technical support and access to Adec’s AR-assisted field service platform for complex deployments.
| Component | Coverage Period | Details |
|---|---|---|
| Frame & Lift Mechanism | 5 years | Includes structural integrity and hydraulic/pneumatic systems |
| Electronics & Control Systems | 3 years | PCB boards, foot controls, touchscreen interfaces |
| Upholstery & Surface Materials | 2 years | Wear, staining, and seam integrity under normal use |
Extended Service Agreements (ESA) are available for up to 10 years, including preventive maintenance, priority response, and software updates. Distributors may bundle ESA with chair sales for enhanced client retention.
© 2026 Professional Dental Equipment Guide | For Authorized Distributors and Healthcare Procurement Officers
Need a Quote for Adec Dental Chair Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


