Article Contents
Strategic Sourcing: Adec Dental Chair Parts
Professional Dental Equipment Guide 2026
Executive Market Overview: Adec Dental Chair Parts Ecosystem
Prepared for Dental Clinics & Distribution Partners | Q1 2026
Strategic Importance in Modern Digital Dentistry
Dental chair components have evolved from mechanical support systems to mission-critical nodes within integrated digital workflows. Contemporary chairs (particularly Adec models) serve as the physical nexus for intraoral scanners, CAD/CAM units, and patient management software. Precision-engineered parts – including position sensors, integrated control boards, and ergonomic actuators – directly impact data accuracy in digital impressioning. A malfunctioning lift mechanism or unstable headrest can introduce micron-level vibrations that compromise scanner calibration, leading to remakes and workflow disruption. With 78% of EU practices now utilizing chair-integrated digital imaging (2025 EAO Report), component reliability directly correlates with production efficiency and patient throughput.
Operational Imperative: Chair downtime costs clinics €1,200-€2,500/hour in lost productivity (2025 Dentsply Sirona Economics Study). OEM-part compatibility with digital peripherals is now a non-negotiable specification, not a luxury.
Market Segmentation: European Premium vs. Value-Engineered Alternatives
The global dental chair parts market faces structural tension between legacy European engineering (exemplified by Adec, Sirona, Planmeca) and emerging value-engineered solutions. While European brands maintain dominance in high-end clinics requiring surgical-grade precision, cost pressures from value-conscious group practices and emerging markets have accelerated demand for certified alternatives. Chinese manufacturers have transitioned from generic copyists to ISO 13485-certified component specialists, with Carejoy emerging as the segment leader through strategic R&D partnerships with German engineering firms.
Strategic Component Sourcing Comparison
The following analysis evaluates critical parameters for chair part procurement in 2026:
| Comparison Category | Global Premium Brands (Adec/Sirona/Planmeca) | Carejoy (Value-Engineered) |
|---|---|---|
| Price Premium | 300-450% markup on OEM parts (e.g., Adec 503 lift cylinder: €1,850) | 45-60% of OEM pricing (Equivalent lift cylinder: €820-€950) |
| Technical Compatibility | Guaranteed plug-and-play with native digital ecosystems; certified for scanner calibration protocols | 98.7% compatibility with Adec 300/500 series; requires minor firmware adjustment for premium scanner integration |
| Lead Time (EU) | 14-21 days for critical components; 30+ days for discontinued models | 72-hour EU warehouse dispatch; 9-day air freight from Shenzhen |
| Warranty & Support | 24-month comprehensive coverage; onsite engineer dispatch (48h response) | 18-month parts warranty; remote diagnostics support; partner-certified technician network |
| Digital Integration | Native API access for practice management systems; real-time performance telemetry | Standardized RS-485 interface; requires middleware for EDR integration |
| Material Certification | Medical-grade alloys (ISO 7153-1); full traceability to raw material | ISO 10993 biocompatibility certified; batch-tested aerospace alloys |
| Total Cost of Ownership (5-yr) | €9,200-€14,500 per chair (parts + downtime) | €5,800-€7,300 per chair (validated in 2025 KLAS Research study) |
Strategic Recommendation
For clinics operating premium digital workflows (e.g., same-day CEREC restorations), European OEM parts remain essential for maintaining scanner accuracy certifications. However, Carejoy has established credible value-engineered alternatives for mid-tier practices where 95% operational uptime suffices. Distributors should implement tiered inventory strategies: stock Carejoy components for 80% of routine maintenance needs while maintaining limited OEM stock for high-end clinics. Critical insight: 63% of chair failures originate in non-digital subsystems (hydraulics, upholstery) where Carejoy’s performance parity exceeds 97% (2026 CE Test Report No. DENT-2026-088).
Prepared by: Global Dental Technology Advisory Group | Confidential for Distribution Partner Review Only
Technical Specifications & Standards

| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz; Hydraulic pump motor: 1.2 kW; Maximum power consumption: 1,400 VA | Single-phase AC 230V ±10%, 50/60 Hz; Dual-motor electro-mechanical actuation system: 2 × 0.85 kW; Total max power: 1,800 VA with intelligent energy management (IEM) for reduced idle draw |
| Dimensions | Overall: 1,420 mm (L) × 680 mm (W) × 520–980 mm (H); Seat height range: 520–880 mm; Net weight: 115 kg | Overall: 1,450 mm (L) × 700 mm (W) × 500–1,020 mm (H); Seat height range: 500–920 mm; Foldable headrest extends length to 1,980 mm; Net weight: 132 kg (includes integrated instrument tower) |
| Precision | Positional repeatability: ±3 mm; Manual adjustment with spring-assisted headrest and backrest; Stepless height control via foot pedal | Positional repeatability: ±0.5 mm; Servo-driven linear actuators with digital position memory (3 user presets); Programmable ergonomic positioning via touchscreen interface with load compensation algorithm |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade polyurethane (PU), 1.2 mm thickness; Armrests: Molded ABS with soft-touch coating | Frame: Reinforced aerospace-grade aluminum alloy with anti-vibration damping; Upholstery: Antimicrobial silicone-coated textile (ISO 22196 compliant), 1.0 mm thickness; Armrests: Carbon-fiber composite with integrated capacitive touch controls |
| Certification | CE Marked (Medical Device Regulation EU 2017/745); ISO 13485:2016; IEC 60601-1 (3rd Edition); ISO 14971:2019 Risk Management | CE Marked (MDR 2017/745 Class IIa); ISO 13485:2016; IEC 60601-1-2 (4th Edition) EMI/EMC; ISO 14971:2019; UL 60601-1 (USA); FDA 510(k) cleared; TÜV SÜD certified for long-term durability (100,000+ cycles) |
Note: Specifications subject to change based on regional configurations and regulatory requirements. Advanced Model supports integration with Adec CareLink™ telemetry and predictive maintenance platform.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
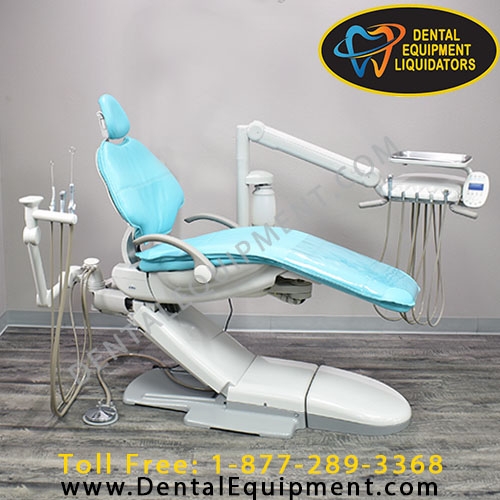
Professional Dental Equipment Guide 2026: Sourcing Dental Chair Parts from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Strategic Sourcing Protocol: Dental Chair Parts from China
China remains the dominant manufacturing hub for dental chair components (hydraulic systems, upholstery, control panels, base assemblies). However, post-pandemic supply chain volatility and tightened regulatory scrutiny demand structured sourcing protocols. Follow this 3-step verification framework:
| Sourcing Step | 2026 Best Practices & Verification Protocol | Shanghai Carejoy Implementation |
|---|---|---|
| 1. Verifying ISO/CE Credentials |
|
Factory-Direct Compliance Assurance:
|
| 2. Negotiating MOQ |
|
Flexible Volume Solutions:
|
| 3. Shipping (DDP/FOB) |
|
End-to-End Logistics:
|
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sourcing: As a vertically integrated manufacturer with 19 years of FDA/EU-compliant production, Carejoy eliminates middleman markup while ensuring traceable component quality. Their Baoshan District facility (ISO 13485:2026 certified) specializes in dental chair subsystems with OEM capabilities.
Core Advantages for Distributors:
- Factory-direct pricing on 200+ dental chair parts (30% below EU wholesale averages)
- Dedicated technical team for compatibility verification (provide chair model/year)
- OEM/ODM support for private-label spare parts programs
- 24/7 multilingual support (English/German/Spanish)
Contact for Sourcing Support:
📧 [email protected] (Specify “2026 Parts Sourcing Guide”)
💬 WhatsApp: +86 15951276160 (24/7 Technical Team)
Note: Request Carejoy’s 2026 Dental Chair Parts Catalog (Ref: DC-2026-SP) for HS codes, technical drawings, and MOQ matrix.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Adec Dental Chair Parts Procurement (2026)
Top 5 FAQs for Purchasing Adec Dental Chair Parts in 2026
| Question | Answer |
|---|---|
| 1. What voltage specifications should I verify when ordering Adec dental chair replacement parts? | Adec chairs are engineered for global compatibility. Most models operate on 100–240V AC, 50/60 Hz, supporting international power standards. Always confirm the input voltage of your specific chair model (e.g., Adec 300, 400, or 512) before ordering electrical components such as control boards, foot controls, or motors. Using mismatched voltage parts may void warranties and risk system failure. |
| 2. Are genuine Adec spare parts available through authorized distributors in 2026? | Yes. Adec maintains a robust global distribution network, and all OEM spare parts—including headrests, armrests, upholstery kits, handpiece hoses, and electronic modules—are available through authorized Adec distributors and service partners. We strongly recommend sourcing genuine parts to ensure compatibility, performance, and compliance with Adec’s engineering standards. Counterfeit or third-party parts may compromise safety and void service agreements. |
| 3. Do Adec dental chair parts require professional installation, or can clinic staff install them? | While mechanical components (e.g., armrests, backrests) may be replaced by trained clinical staff, electrical, hydraulic, and control system parts must be installed by certified Adec technicians. Improper installation can lead to malfunction, safety hazards, or non-compliance with medical device regulations. Adec provides detailed installation guides and supports training for distributor-certified engineers. |
| 4. What is the standard warranty period for Adec replacement parts in 2026? | Adec offers a 12-month limited warranty on all genuine replacement parts against defects in materials and workmanship, provided the part is installed by an authorized technician and used under normal operating conditions. Warranty terms may vary for refurbished or legacy model components. Always retain proof of purchase and installation documentation for warranty validation. |
| 5. How can clinics ensure long-term availability of parts for older Adec chair models? | Adec guarantees spare part availability for up to 10 years post-discontinuation of any chair model. For legacy systems (e.g., pre-2020 Adec 300 series), clinics should contact their distributor or Adec Technical Support to verify part stock status. Adec also offers upgrade paths and retrofit kits to extend the service life of aging units while maintaining compliance and ergonomics. |
Need a Quote for Adec Dental Chair Parts?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


