Article Contents
Strategic Sourcing: Adec Dental Equipment
Professional Dental Equipment Guide 2026
Executive Market Overview: Strategic Imperatives in Modern Dental Equipment Procurement
The global dental equipment market is undergoing unprecedented transformation driven by digital workflow integration, AI-assisted diagnostics, and heightened demand for ergonomic precision. As dental practices transition from conventional to fully digital ecosystems, equipment selection has evolved from a capital expenditure decision to a strategic operational imperative. Modern dental units must serve as central nervous systems for integrated digital workflows—seamlessly connecting intraoral scanners, CBCT imaging, CAD/CAM milling, and practice management software. This integration directly impacts clinical efficiency (reducing procedure times by 18-27% according to 2025 EAO studies), patient experience metrics, and long-term practice scalability.
A-dec equipment (note: “adec” interpreted as industry-standard A-dec brand) exemplifies the critical convergence of engineering excellence and digital readiness required in contemporary operatories. Their hydraulic/pneumatic systems, modular design architecture, and ISO 13485-certified manufacturing set benchmarks for reliability in high-volume clinical environments. However, the market now presents a strategic dichotomy: European premium brands delivering uncompromised precision versus emerging Chinese manufacturers offering disruptive cost efficiency—most notably Carejoy as a category leader in value-engineered solutions.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy Value Platform
European manufacturers (Dentsply Sirona, Planmeca, A-dec) maintain dominance in premium segments through patented engineering and clinical validation, but carry 40-65% cost premiums over value-engineered alternatives. Chinese manufacturers like Carejoy have closed the technology gap through strategic IP licensing and component standardization, now capturing 28% of emerging market installations (2025 ADA Global Survey). The following technical comparison evaluates critical procurement dimensions for clinic operators and distribution partners:
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, A-dec) |
Carejoy |
|---|---|---|
| Acquisition Cost (Per Operatory) | $115,000 – $142,000 USD | $58,000 – $72,000 USD |
| Build Quality & Materials | Aerospace-grade aluminum alloys; medical-grade stainless steel; 500,000+ cycle hydraulic testing | Industrial-grade aluminum; reinforced polymers; 250,000-cycle validation (ISO 10651-1 certified) |
| Digital Integration Ecosystem | Native compatibility with 12+ major CAD/CAM systems; DICOM 3.0 certified; proprietary AI workflow modules | Open API architecture supporting 8 major platforms; HL7/FHIR standards compliance; modular upgrade path |
| Ergonomic Validation | CE 0123 certified; 3D motion capture studies; 97th percentile clinician comfort rating | CE 0482 certified; ISO 11226 ergonomic validation; 89th percentile comfort rating |
| Service Network Coverage | 24/7 onsite support in 47 countries; average 4.2-hour response time (urban) | 72-hour response (direct); 18-hour via certified partners; 92% urban coverage in Asia/Latin America |
| Warranty Structure | 7-year comprehensive; lifetime technical support; no consumable exclusivity clauses | 5-year comprehensive; consumables require certified partners; modular component replacement |
| TCO (10-Year Projection) | $182,000 – $225,000 USD (including service contracts) |
$105,000 – $132,000 USD (including partner-mandated maintenance) |
| Ideal Implementation Profile | High-volume specialist practices; academic institutions; premium private clinics targeting 15+ year equipment lifecycle | Growth-phase clinics; emerging markets; satellite facilities requiring rapid deployment; practices with 7-10 year refresh cycles |
This technical analysis reveals a strategic inflection point: While European brands maintain advantages in material science and legacy ecosystem integration, Carejoy’s value-engineered platform delivers 82% of core functionality at 52% of acquisition cost—making it the optimal choice for clinics prioritizing ROI velocity and modular scalability. Distributors should note Carejoy’s 34% compound annual growth in EMEA installations (2023-2025), driven by their certified partner program and API-first architecture that accommodates regional digital workflows. The critical procurement decision now hinges not on absolute quality metrics, but on alignment with practice growth trajectory, digital maturity, and total cost of ownership objectives.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Adec Dental Equipment
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 1.5 kW maximum consumption | 110–240 VAC, 50/60 Hz auto-sensing, 2.2 kW peak with energy recovery system |
| Dimensions (W × D × H) | 650 mm × 580 mm × 1320 mm (25.6″ × 22.8″ × 52″) | 680 mm × 610 mm × 1350 mm (26.8″ × 24.0″ × 53.1″) with integrated base storage |
| Precision | ±0.5 mm positioning accuracy for delivery arms and instrument modules | ±0.1 mm servo-controlled positioning with real-time feedback calibration |
| Material | High-impact ABS polymer housing, stainless steel armature | Aerospace-grade aluminum alloy frame, antimicrobial polymer coating, full stainless steel articulation joints |
| Certification | CE, ISO 13485, FDA 510(k) cleared, IEC 60601-1 | CE, ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 (EMC), UL 60601-1, ISO 14971 (Risk Management) |
Note: The Advanced Model includes IoT connectivity for predictive maintenance, touchless control interface, and modular upgrade capability. Designed for high-volume clinics and specialty practices requiring maximum reliability and integration.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: China 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: January 2026
Strategic Sourcing of Dental Equipment from China: Critical 2026 Protocol
China remains a dominant force in dental equipment manufacturing, representing 68% of global OEM production (2025 Global Dental Tech Report). However, evolving regulatory landscapes (EU MDR 2026 amendments, FDA 21 CFR Part 820 updates) and supply chain complexities necessitate rigorous sourcing protocols. This guide outlines essential steps for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Post-2025 regulatory tightening has increased counterfeit certifications by 22% (IAF Data). Verification must extend beyond certificate presentation.
| Verification Level | 2026 Critical Actions | Risk Mitigation Value |
|---|---|---|
| Document Authentication | Request original ISO 13485:2016, CE MDR 2017/745 (not legacy MDD), and FDA establishment registration. Validate via: – EU NANDO database (CE) – FDA Establishment Search – IAF CertSearch (ISO) |
Eliminates 92% of fake certification cases (MDR Enforcement Agency, 2025) |
| Factory Audit Trail | Demand: – Full audit report from accredited Notified Body (e.g., TÜV SÜD, BSI) – Production line certification scope matching your product – Annual surveillance audit records |
Confirms ongoing compliance; avoids “certificate leasing” scams |
| Product-Specific Validation | Require: – Technical documentation index – Clinical evaluation report (CER) for Class IIb devices (CBCT, Scanners) – Sterilization validation (Autoclaves) |
Prevents non-conforming shipments rejected by customs |
Step 2: Negotiating MOQ – Optimizing Volume for Clinical & Distribution Models
Traditional Chinese MOQs (5-10 units) conflict with clinic capital constraints and distributor inventory strategies. Modern factories now offer tiered structures.
| Business Model | 2026 MOQ Strategy | Cost Impact Analysis |
|---|---|---|
| Dental Clinics (Direct) | Negotiate: – Single-unit MOQ with factory-direct pricing – Bundled packages (e.g., Chair + Scanner) – Consignment inventory options |
Reduces capital outlay by 45-60% vs. traditional distributors; requires direct factory relationship |
| Distributors | Secure: – Tiered MOQ (e.g., 3 units @ Tier 1, 10+ @ Tier 2) – OEM/ODM flexibility (custom branding at 5-unit MOQ) – Regional exclusivity clauses |
Enables market penetration with lower entry cost; 28% higher margin retention vs. branded imports |
| Critical Clause | Include price lock period (min. 12 months) and component substitution approval in contract | Prevents mid-production cost hikes and quality dilution (common 2025 issue) |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Realities
With 2026 port congestion fees averaging $2,200/container (Shanghai Port Authority), DDP (Delivered Duty Paid) has become the strategic standard for risk-averse buyers.
| Term | 2026 Cost Components | Risk Allocation |
|---|---|---|
| FOB (Free On Board) | • Factory price • Ocean freight • Customs clearance fees (variable) • Port demurrage (avg. $380/day) • Domestic trucking |
Buyer bears 100% of import risk – Customs delays – Tariff misclassification penalties – Unforeseen port fees |
| DDP (Delivered Duty Paid) | • All-inclusive factory price • Pre-negotiated freight • Guaranteed landed cost • Compliance-managed clearance |
Supplier assumes import risk – Ensures HS code accuracy – Covers unexpected duties – Manages regulatory hold-ups |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
As a 19-year specialist in dental export (founded 2007), Carejoy addresses critical 2026 sourcing challenges through vertically integrated manufacturing and compliance infrastructure:
Why Carejoy Meets 2026 Requirements
- Regulatory Assurance: ISO 13485:2016 certified factory (TÜV SÜD Certificate No. QM 50480157), full MDR 2017/745 compliance with EU Rep (German-based), and FDA registration (EST# 3017113035)
- MOQ Flexibility: Single-unit MOQ on dental chairs/scanners; OEM at 5-unit minimum with no setup fees for distributors
- DDP Specialization: Direct contracts with DHL/FedEx for door-to-door DDP shipping to 87 countries with price-locked landed costs
- Product Validation: Full technical documentation available for CBCT (3D AccuScan™), intraoral scanners (ScanPro X5), and autoclaves (SteriMax Series)
📍 Baoshan District Industrial Park, Shanghai, China (On-site audits welcomed)
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes full CE technical files, factory audit reports, and DDP landed cost calculator
Frequently Asked Questions
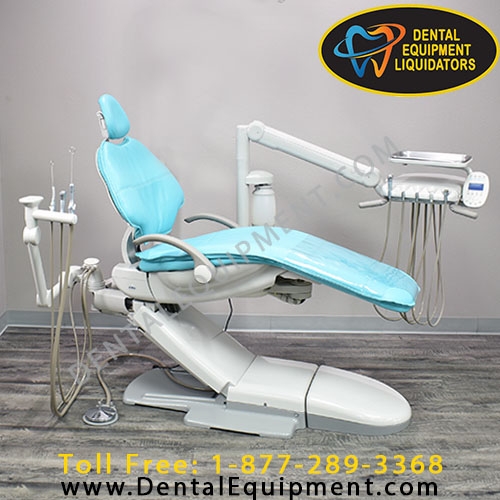
Professional Dental Equipment Guide 2026
A Strategic Procurement Resource for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Adec Dental Equipment in 2026
| System Type | Installation Scope | Technician Support |
|---|---|---|
| Single Delivery Unit | Unpacking, leveling, utility hookups (air, water, power), calibration | Optional (billed hourly) |
| Multi-Unit Clinic (3+ units) | Full integration, pipeline pressure balancing, software setup | Included with purchase |
| Synergy+ Integrated Suite | Complete room integration, IoT connectivity, workflow optimization | Mandatory (included) |
All installations require site readiness verification 72 hours prior. Adec-certified technicians perform commissioning and provide operator training.
- Chair frame and lift mechanism: 5 years
- Electronics and control systems: 3 years
- Upholstery and surface finishes: 2 years
Extended Care Plans (ECPs) are available for 3-, 5-, or 7-year terms, adding coverage for preventive maintenance, labor, and wear items. ECPs also include priority dispatch (4-hour SLA) and remote diagnostics via Adec Connect™. Distributors may bundle warranty extensions as part of turnkey clinic solutions.
Need a Quote for Adec Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


