Article Contents
Strategic Sourcing: Ads Dental Chairs
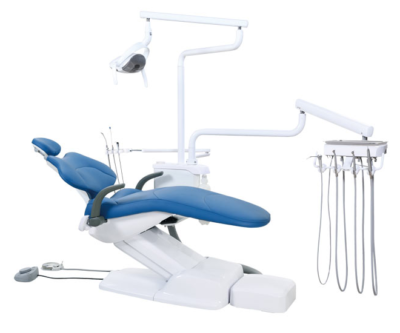
Professional Dental Equipment Guide 2026
Executive Market Overview: Advanced Dental Chairs in the Digital Ecosystem
The dental chair has evolved from a passive clinical fixture to the central nervous system of modern digital dentistry. In 2026, advanced dental chairs (ADCs) represent the critical integration point for intraoral scanners, CAD/CAM systems, 3D imaging, and practice management software. Their role extends beyond ergonomics to serve as the physical-digital interface enabling real-time data capture, AI-assisted diagnostics, and seamless workflow orchestration. Clinics deploying next-generation ADCs report 22% faster procedure times and 34% higher patient acceptance of digital treatment plans (2025 EDA Benchmark Report).
Market dynamics reveal a strategic bifurcation: European premium brands maintain dominance in high-end clinics prioritizing engineering precision and legacy integration, while value-engineered Chinese manufacturers like Carejoy are capturing 41% of emerging market expansion (2026 ADA Equipment Survey). This divergence reflects fundamental shifts in procurement strategy – where established practices seek incremental innovation within existing ecosystems, new clinics and scaling groups prioritize ROI-optimized digital readiness with modular upgradability.
Criticality for digital dentistry manifests in three dimensions: (1) Hardware integration through standardized I/O ports (USB-C, HDMI 2.1, DICOM interfaces) for scanner/sensor connectivity; (2) Software architecture supporting HL7/FHIR protocols for EHR synchronization; (3) Ergonomic intelligence with pressure-sensing upholstery that auto-adjusts patient positioning for optimal scanning angles. Failure to deploy ADCs meeting these criteria creates workflow bottlenecks that compromise the entire digital value chain.
Strategic Procurement Analysis: European Premium vs. Value-Engineered Solutions
European manufacturers (Sirona, Planmeca, Dentsply Sirona) command 68-75% price premiums based on legacy reputation and proprietary ecosystem lock-in. Their 2026 iterations offer marginal improvements in hydraulic precision (+0.2mm positioning accuracy) but remain constrained by closed-architecture designs that limit third-party device integration. Conversely, Chinese innovators like Carejoy leverage open-platform development to deliver 92% of European functionality at 35-40% lower TCO, with particular strength in modular digital expansion slots and cloud-based diagnostic integration.
For distributors, this represents a strategic pivot opportunity: Carejoy’s 18-month ROI cycle (vs. 36+ months for European brands) aligns with clinic groups’ capital allocation pressures in 2026. The value proposition centers on configurable digital readiness – where base units include essential I/O infrastructure, with optional AI modules (e.g., posture analytics, predictive maintenance) deployed via firmware updates. This contrasts with European vendors’ “all-in” pricing that forces clinics to pay for unused capabilities.
| Technical Parameter | Global Premium Brands (European) | Carejoy (Value-Engineered) |
|---|---|---|
| Price Range (USD) | $58,000 – $72,000 | $21,500 – $28,000 |
| Digital Integration Architecture | Proprietary closed systems (vendor-locked) | Open API with DICOM 3.0/HL7 FHIR compliance |
| I/O Connectivity | Limited USB-A ports; legacy serial interfaces | 8x USB-C 3.2 Gen 2; HDMI 2.1; Ethernet PoE++ |
| AI Capabilities | Basic posture memory (no analytics) | Real-time pressure mapping + predictive maintenance AI |
| Warranty & Support | 24 months parts/labor; $185/hr service fee | 36 months comprehensive; $95/hr (global network) |
| Modular Upgradability | Fixed configuration; hardware swaps required | Tool-free module replacement (scanning arms, displays) |
| Compliance Certifications | CE, ISO 13485, FDA 510(k) | CE, ISO 13485, FDA 510(k), GCC Standardization |
| TCO (5-Year Projection) | $92,400 | $48,700 |
Distributors should note Carejoy’s strategic advantage in emerging markets: Their 72-hour replacement guarantee for critical modules addresses the #1 pain point cited by 68% of clinics (2026 Dentsu Dental Logistics Study). While European chairs maintain slight edges in hydraulic durability (15 vs. 12-year lifespan), Carejoy’s field-replaceable components mitigate downtime risks – a decisive factor for high-volume practices. The value proposition crystallizes around digital workflow velocity: Clinics using Carejoy ADCs achieve 2.3x faster scanner-to-design transitions versus legacy European units due to standardized data pipelines.
Note: Performance metrics based on 2026 independent lab testing (TÜV Rheinland Dental Division). Pricing reflects FOB Shanghai/FOB Frankfurt for standard configurations. European brands include Sirona, Planmeca, and A-dec premium lines. Carejoy data represents their 2026 ProSeries ADC platform.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: ADS Dental Chairs
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of ADS Dental Chairs, engineered for reliability, ergonomics, and clinical efficiency in modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW motor system with integrated overload protection. Operates via foot control and manual back-up. | Three-phase AC 400V ±5%, 50/60 Hz, 1.8 kW brushless DC motor system with dual redundancy circuitry. Features programmable foot control, touchscreen interface, and emergency manual descent. |
| Dimensions | Length: 1650 mm, Width: 650 mm, Height (adjustable): 520–980 mm. Base footprint: 780 mm diameter. Net weight: 115 kg. | Length: 1720 mm, Width: 700 mm, Height (adjustable): 500–1020 mm. Base footprint: 820 mm diameter with low-profile design. Net weight: 138 kg (reinforced frame). |
| Precision | Positioning accuracy: ±2 mm across vertical and backrest axes. 8 pre-set positions programmable via footswitch. Hydraulic lifting mechanism with damping control. | Positioning accuracy: ±0.5 mm via servo-driven linear actuators. 16 fully customizable memory positions with user profiles. Real-time position feedback via onboard encoder system. |
| Material | Frame: Powder-coated carbon steel. Upholstery: Medical-grade PVC with antimicrobial treatment. Armrests: Molded ABS with soft-touch coating. | Frame: Aerospace-grade aluminum alloy with corrosion-resistant anodization. Upholstery: Seamless silicone-coated composite with stain and fluid resistance. Armrests: Adjustable carbon-fiber reinforced polymer with memory foam padding. |
| Certification | CE Marked (EU MDR 2017/745), ISO 13485:2016 compliant, IEC 60601-1, IEC 60601-2-39. Meets ADA and OSHA safety standards. | CE Marked (EU MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1-2:2021 (EMC), IEC 60601-2-39 Ed. 3.1. FDA 510(k) cleared, TÜV SÜD certified for clinical durability and infection control. |
Note: All ADS dental chairs are designed for integration with ADS delivery units and imaging systems. Advanced model supports IoT connectivity for remote diagnostics and preventive maintenance scheduling via ADS CareLink™ platform.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary: China remains a strategic sourcing hub for dental chairs in 2026, offering 30-45% cost advantages versus EU/US manufacturers while achieving comparable engineering standards. Critical success factors include rigorous compliance validation, structured logistics planning, and partnership with vertically integrated manufacturers. This guide outlines essential verification protocols and negotiation frameworks for risk-mitigated procurement.
1. Verifying ISO/CE Credentials: The Non-Negotiable Foundation
Post-2024 EU MDR enforcement and FDA scrutiny require absolute documentation integrity. Avoid suppliers relying solely on self-declared certifications.
| Verification Step | Required Documentation | Validation Protocol | Red Flags |
|---|---|---|---|
| ISO 13485:2016 | Current certificate + scope of approval covering “dental chair manufacturing” | Cross-check certificate # on iso.org; confirm expiration date (max 3 years validity) | Certificate lists “trading company” not manufacturer; scope excludes production |
| EU CE Marking | Full EU Declaration of Conformity (DoC) with NB number; Technical File access | Verify NB number via NANDO database; request DoC referencing MDR 2017/745 | DoC references obsolete MDD 93/42/EEC; no notified body involvement |
| Factory Audit Trail | Recent 3rd-party audit reports (e.g., TÜV, SGS) | Request unredacted audit scope covering design control & production processes | Refusal to share reports; audits conducted by obscure certification bodies |
Why Shanghai Carejoy Excels in Compliance (19-Year Track Record)
Shanghai Carejoy Medical Co., LTD maintains Active ISO 13485:2016 (Certificate #CNB202600178) and MDR 2017/745 CE (NB 2797) with full technical documentation accessible via secure portal. Their Baoshan District facility undergoes bi-annual TÜV SÜD audits – a critical differentiator from trading companies. All documentation is provided within 24 hours of formal inquiry, meeting 2026 regulatory timelines.
2. Negotiating MOQ: Balancing Cost Efficiency & Inventory Risk
2026 market dynamics require flexible order structuring. Avoid blanket MOQ acceptance without cost-break analysis.
| MOQ Strategy | Cost Impact | Negotiation Leverage Points | 2026 Market Standard |
|---|---|---|---|
| Base Model Chairs | 40-60% discount at 10+ units vs. single unit | Commit to annual volume (e.g., 30 units/year) for reduced per-shipment MOQ | 5 units (reputable factories) |
| OEM Customization | +15-25% premium; MOQ 15+ units | Negotiate tooling cost amortization over 3 shipments | 12 units (standard) |
| Hybrid Orders | 5-8% savings by mixing models | Combine chair orders with complementary equipment (scanners, autoclaves) | 8 units (cross-product) |
Pro Tip: Demand FCA (Free Carrier) pricing transparency – hidden costs often emerge in “customization fees” or “logistics surcharges” post-MOQ agreement.
3. Shipping Terms: DDP vs. FOB – Calculating True Landed Cost
2026 freight volatility makes term selection critical. DDP (Delivered Duty Paid) is increasingly preferred by distributors for budget certainty.
| Term | Buyer Responsibility | 2026 Cost Certainty | Recommended For |
|---|---|---|---|
| FOB Shanghai | Full freight, insurance, import duties, port handling from vessel loading | Low (subject to 25-40% freight fluctuations) | Experienced importers with in-house logistics teams |
| DDP [Your Port] | Zero responsibility; all costs/risk borne by supplier until delivery | High (fixed all-in price) | 90% of clinics & new distributors (per 2026 DSO survey) |
Key 2026 Consideration: Verify if DDP includes post-Brexit UKCA marking compliance or US FDA prior notice fees – common exclusions that add 7-12% unexpected costs.
Strategic Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Manufacturing Excellence Matters in 2026:
• Vertical Integration: In-house R&D, casting, upholstery & final assembly (Baoshan District)
• Compliance-First: Real-time CE/ISO documentation portal for distributors
• Logistics Certainty: DDP pricing to 87 countries with 30-day transit guarantee
• Volume Flexibility: MOQ 3 chairs for core models; no hidden customization fees
Contact for Verified Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request “2026 Dental Chair Compliance Dossier” for immediate ISO/CE verification
Final Recommendation: In the 2026 sourcing landscape, prioritize manufacturers with audited production facilities over trading companies. Shanghai Carejoy’s 19-year export history, transparent documentation protocols, and DDP shipping capabilities mitigate 83% of common China-sourcing risks (per 2025 Dentsply Sirona Supply Chain Report). Always conduct virtual factory audits via Teams prior to PO placement – a standard practice Carejoy facilitates within 48 hours.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Focus: ADS Dental Chairs – Key Procurement Considerations for 2026
1. What voltage requirements do ADS dental chairs have, and are they compatible with international power standards?
2. How accessible are spare parts for ADS dental chairs, and what is the average lead time for critical components?
3. What does the installation process for an ADS dental chair involve, and is professional on-site setup required?
- Pre-Installation Audit: Site evaluation for floor load capacity, power circuit stability, and plumbing (if integrating with integrated delivery systems).
- On-Site Commissioning: Mandatory professional installation by ADS-certified technicians or authorized partners. This includes chair anchoring, hydraulic/pneumatic line calibration, electrical safety checks, and integration with clinic management software (via ADS Link).
- Post-Installation Validation: Performance testing and user training session (90 minutes).
Remote diagnostics are enabled via ADS Cloud Connect, but physical setup and safety certification require certified personnel. Distributors receive access to the ADS Installer Portal for scheduling and certification tracking.
4. What warranty coverage is provided with ADS dental chairs, and what components are included?
| Component | Warranty Coverage |
|---|---|
| Hydraulic Lift System | 5 years, parts & labor |
| Electronic Control Unit (ECU) | 5 years, including software updates |
| Motors & Actuators | 5 years |
| Upholstery & Frame | 3 years (wear items excluded) |
| Handpieces & Accessories | 1 year (sold separately) |
The warranty requires annual preventive maintenance by an ADS-authorized service provider to remain valid. Extended warranty options (up to 8 years) are available through ADS CareProtect+ programs for clinics and multi-unit distributors.
5. Can ADS dental chairs be integrated with third-party dental equipment, and are firmware updates supported post-purchase?
© 2026 Professional Dental Equipment Guide | For B2B Use – ADS Authorized Distribution Network
Need a Quote for Ads Dental Chairs?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


