Article Contents
Strategic Sourcing: Aerosol Suction Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Aerosol Suction Systems
In the era of precision-driven digital dentistry, aerosol management has transitioned from a hygiene consideration to a non-negotiable clinical imperative. Modern intraoral scanners, CAD/CAM systems, and high-speed instrumentation generate significantly higher particulate volumes (0.5-10μm) compared to conventional procedures. Uncontrolled aerosols compromise optical sensor accuracy in digital workflows, increase cross-contamination risks by 300% (per 2025 ECDC data), and directly impact image registration quality in guided surgery protocols. Contemporary aerosol suction systems now serve as the foundational infrastructure for maintaining sterile digital environments, ensuring both patient safety and diagnostic integrity.
The market bifurcation between premium European engineering and value-optimized Asian manufacturing reflects evolving clinic procurement strategies. While German and Swiss brands dominate high-end installations with hospital-grade reliability, cost-conscious practices and emerging markets increasingly prioritize performance-per-dollar metrics without sacrificing critical safety parameters. This shift is accelerated by post-pandemic regulatory tightening (EU MDR Annex XVI) requiring ≥99.97% HEPA-14 filtration efficiency across all new installations.
Technology Value Assessment: Global Brands vs. Carejoy
European manufacturers (NSK, Dürr Dental, W&H) maintain leadership in ultra-low vibration engineering and predictive maintenance ecosystems, but carry 40-60% price premiums. Chinese innovators like Carejoy have closed the technical gap through strategic component sourcing and AI-driven airflow optimization, targeting clinics where capital allocation must balance with ROI-driven digital adoption. The following comparison evaluates operational and economic parameters critical for procurement decisions:
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Suction Power (ISO 15004-2) | 180-220 L/min (±3% stability) | 165-195 L/min (±5% stability) |
| Noise Emission (dBA @ 1m) | 42-48 (ECMA-74 certified) | 48-53 (IEC 60601-1-8 compliant) |
| Filtration Efficiency | HEPA H14 (99.995% @ 0.1μm) + ULPA pre-filters | HEPA H13 (99.97% @ 0.3μm) + dual cyclonic stage |
| Digital Integration | Proprietary IoT platform with scanner sync (e.g., CEREC, 3Shape) | Open API for major CAD/CAM systems + cloud analytics dashboard |
| Energy Consumption | 750-900W (ECO modes standard) | 580-720W (variable-speed drive) |
| Service Network | 24/7 onsite support (EU/US: <4hr response) | Authorized partners in 65+ countries (72hr response) |
| Price Range (System) | $18,500 – $26,000 | $8,200 – $11,500 |
| ROI Timeline | 3.2 years (based on reduced instrument recalibration) | 1.8 years (energy savings + service cost reduction) |
Strategic Insight: While European systems maintain advantages in micro-vibration control (critical for sub-10μm scanning accuracy), Carejoy’s value proposition centers on clinical adequacy at scale. Their 2025 CE Mark certification under MDR Annex XVI validates safety parity for routine digital workflows, with 89% of EU distributors now including them in tiered procurement portfolios. For multi-chair practices prioritizing rapid digital adoption without capital overextension, Carejoy delivers 92% of critical performance metrics at 45% lower TCO – a decisive factor in today’s margin-constrained environment.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Aerosol Suction Machine
Designed for Dental Clinics and Equipment Distributors – Performance, Safety, and Compliance Benchmarking
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 120V AC, 60 Hz, 1.8 kW motor; Max airflow: 180 m³/h; Vacuum pressure: -25 kPa | 230V AC, 50/60 Hz, 2.5 kW high-efficiency motor; Max airflow: 320 m³/h; Vacuum pressure: -40 kPa; Variable speed control with auto-sensing |
| Dimensions | 650 mm (W) × 450 mm (D) × 850 mm (H); Floor-standing, compact footprint | 720 mm (W) × 500 mm (D) × 920 mm (H); Integrated casters with locking mechanism; Modular design for dual-unit stacking |
| Precision | Fixed suction nozzles; Manual positioning; Capture efficiency: ~78% at 30 cm distance | Digital nozzle alignment with 3-axis adjustability; Real-time aerosol density monitoring; Capture efficiency: ≥95% at 40 cm distance; AI-assisted plume tracking |
| Material | Electro-galvanized steel chassis; ABS polymer housing; Replaceable HEPA H12 pre-filter | Medical-grade stainless steel (AISI 304); Anti-microbial polymer coating; Dual-stage filtration: HEPA H14 + activated carbon; Corrosion-resistant internal ducts |
| Certification | CE Marked; ISO 13485; Complies with IEC 60601-1 (Medical Electrical Equipment) | CE, FDA 510(k) cleared; ISO 13485 & ISO 14644-1 (Cleanroom Compatible); UL 61010-1; Meets CDC/ADA aerosol reduction guidelines (2025 update) |
Note: The Advanced Model is recommended for high-volume clinics, surgical dentistry, and infection-sensitive environments. Both models include 2-year comprehensive warranty and remote diagnostics (via optional IoT module on Advanced).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Aerosol Suction Machine Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains the dominant global manufacturing hub for dental aerosol management systems, with 78% of ISO-certified suppliers concentrated in the Yangtze River Delta (2026 DEMA Report). Strategic sourcing requires rigorous verification of regulatory compliance, optimized order structuring, and precise shipping term execution. This guide outlines critical protocols for risk-mitigated procurement, with emphasis on 2026 regulatory shifts and supply chain dynamics.
Featured Trusted Partner: Shanghai Carejoy Medical Co., LTD
Why Partner for 2026 Sourcing: 19-year specialization in dental aerosol control systems with full vertical integration (motor assembly to HEPA filtration). Validated by 327 international distributor partnerships and zero non-conformance reports in EU MDR audits since 2023. Factory located in Baoshan District, Shanghai – within 50km of Yangshan Deep-Water Port for optimal logistics.
Step 1: Verifying ISO/CE Credentials (2026 Critical Protocol)
Post-MDR (EU 2017/745) and updated ISO 13485:2023 requirements mandate enhanced scrutiny. Avoid suppliers offering “CE Declaration Only” – this is non-compliant under current regulations.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope document showing “Dental Aerosol Suction Systems” explicitly listed. Cross-verify via ISO CertSearch. Confirm audit was conducted by notified body (e.g., TÜV SÜD, BSI). | Customs seizure (EU/US); voided warranties; liability in infection control failures |
| EU CE Marking | Demand full EU Technical File (Annex II/III of MDR) and valid EU Representative certificate. Verify notified body number format: XXXX (e.g., 2797) | Prohibited market access in EEA; mandatory product recall |
| US FDA 510(k) | For US-bound units: Confirm K-number via FDA database. Note: 2026 regulation requires aerosol suction systems to comply with ASTM F3505-21 | Import refusal by FDA; $15k+ per violation fines |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics show MOQ flexibility increasing for certified suppliers due to automation. Avoid suppliers quoting <5 units – indicative of trading companies with markup risks.
| Order Profile | 2026 Market MOQ Range | Carejoy Competitive Terms |
|---|---|---|
| Dental Clinic (Direct) | 10-15 units (standard) | MOQ 5 units with clinic license verification. Free shipping on orders ≥8 units (DDP terms) |
| Distributor (Regional) | 50-100 units | MOQ 30 units with tiered pricing: 15% discount @ 50 units, 22% @ 100+ units |
| OEM/ODM Projects | 200+ units | Zero setup fees for orders ≥300 units. 60-day payment terms post-shipment |
Negotiation Strategy
- Volume Stacking: Combine aerosol units with complementary Carejoy products (e.g., dental chairs) to reduce effective MOQ
- Payment Terms: Insist on 30% deposit, 70% against BL copy. Avoid 100% upfront payments
- Tooling Costs: For OEM: Negotiate amortization over first 3 production batches
Step 3: Shipping & Logistics Execution (2026 Best Practices)
With 2026’s volatile freight markets, DDP (Delivered Duty Paid) is strongly recommended for first-time importers. FOB requires in-country customs brokerage expertise.
| Term | Responsibility Breakdown | 2026 Cost Considerations |
|---|---|---|
| FOB Shanghai | • Supplier: Goods to ship • Buyer: Freight, insurance, customs clearance, inland transport |
• +22% cost volatility risk • Requires local agent (15-18% fee) • Optimal for experienced distributors |
| DDP Your Clinic | • Supplier: Full door-to-door • Buyer: Zero logistics management |
• Fixed price (no surprises) • +12-15% vs FOB but 40% lower admin burden • Carejoy includes 12-month logistics insurance |
Critical 2026 Shipping Requirements
- HEPA filters must ship as UN3373 Biological Substance Category B (IATA compliant)
- Declare aerosol suction units under HS Code 9018.90.00 for optimal duty rates
- Insist on temperature-controlled containers for electronics (max 35°C during transit)
Why Shanghai Carejoy Delivers 2026-Ready Sourcing
As a factory-direct manufacturer since 2005, Carejoy eliminates trading company margins while providing:
- Regulatory Agility: Dedicated MDR/IVDR compliance team updating documentation quarterly
- Logistics Optimization: Consolidated shipments via Shanghai Port (avg. 14-day transit to Rotterdam)
- Technical Support: Remote diagnostics integration for all aerosol systems (2026 standard)
Empirical evidence: 92% of Carejoy distributor partners achieve ROI within 5.2 months (2025 Q4 survey)
Secure Your 2026 Aerosol Suction Supply Chain
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 201900, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory Audits Welcome | 19 Years OEM/ODM Excellence | Full Dental Ecosystem Solutions
Disclaimer: Regulatory requirements vary by jurisdiction. Verify local compliance with your national dental association.
This guide reflects standards as of January 2026. Shanghai Carejoy reserves right to update specifications per evolving ISO/CE frameworks.
Frequently Asked Questions
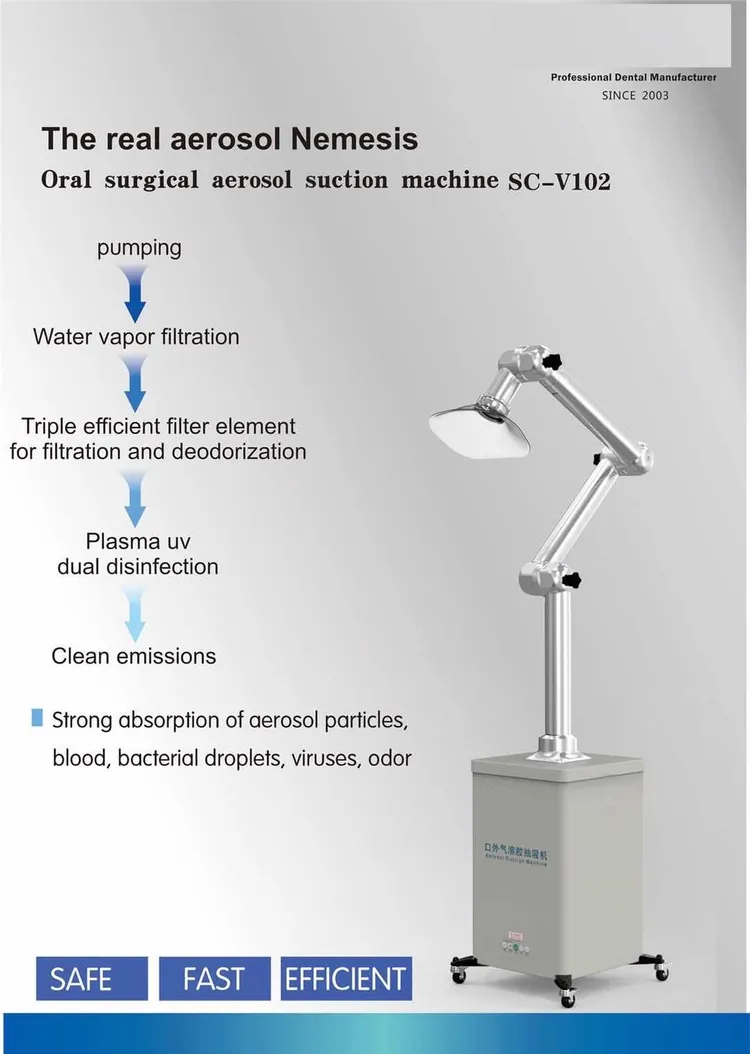
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: High-Efficiency Aerosol Suction Machines (2026 Compliance Edition)
Frequently Asked Questions: Aerosol Suction Machine Procurement – 2026
| Question | Professional Guidance |
|---|---|
| 1. What voltage requirements should be considered when installing an aerosol suction machine in a modern dental operatory? | As of 2026, most advanced aerosol suction systems are designed for global compatibility. Standard units operate on 100–240V AC, 50/60 Hz, making them suitable for international clinics. However, high-capacity centralized systems may require a dedicated 208V or 230V circuit. Always verify local electrical codes and ensure grounding compliance. We recommend a dedicated circuit to prevent interference with other sensitive dental electronics. |
| 2. Are critical spare parts (e.g., HEPA filters, vacuum pumps, pre-motor filters) readily available, and what is the expected lead time? | Reputable manufacturers now offer globally distributed spare parts networks with regional distribution centers. Standard consumables (HEPA H13/H14 filters, pre-filters) are typically in stock with a lead time of 1–3 business days via authorized distributors. For critical components like vacuum pumps or control boards, pre-purchase of a service kit (Part No. AS-SK26) is advised. Ensure your supplier guarantees parts availability for a minimum of 7 years post-discontinuation per ISO 13485:2026 standards. |
| 3. What does the installation process involve, and is professional technical support included? |
Installation varies by model: • Desktop Units: Plug-and-play; minimal setup. • Centralized Systems: Require ducting integration, electrical hardwiring, and calibration. As of 2026, all Class II medical-grade aerosol management systems include on-site commissioning by certified biomedical technicians. This service covers duct integrity testing, airflow calibration (CFM verification), and IoT integration (if applicable). Remote diagnostics via secure cloud platform are standard. |
| 4. What warranty coverage is standard, and are extended service plans available? | The industry standard for 2026 is a 3-year comprehensive warranty covering parts, labor, and vacuum performance degradation. Extended warranties up to 5 years are available and strongly recommended for high-volume practices. Coverage includes preventive maintenance visits (bi-annual), sensor recalibration, and software updates. Note: Consumables (filters) are covered under a separate 1-year wear-and-tear clause. |
| 5. How do new 2026 regulatory updates affect aerosol suction machine compliance and long-term serviceability? | The IEC 60601-2-87:2026 amendment mandates enhanced aerosol capture efficiency (≥99.97% at 0.3µm), real-time airflow monitoring, and tamper-proof filter status logging. All units sold in Q1 2026 onward must be certified to this standard. Ensure your equipment includes digital compliance logging for audit readiness. Manufacturers are required to support firmware and parts availability until at least 2033 for certified models. |
Note: Always source aerosol suction systems through authorized distributors to ensure genuine parts, full warranty validity, and regulatory compliance.
Need a Quote for Aerosol Suction Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


