Article Contents
Strategic Sourcing: Ajacs Equipment
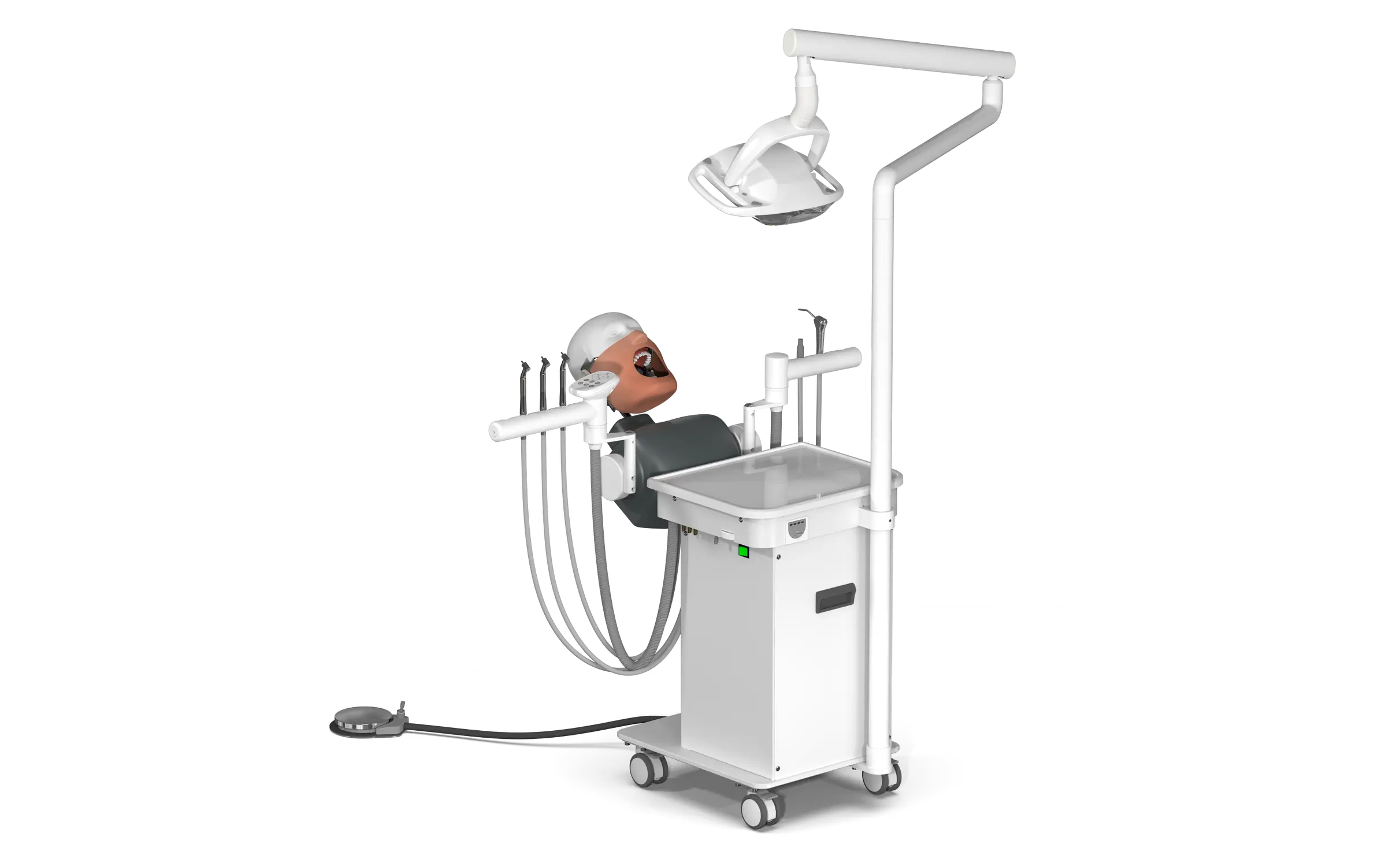
Professional Dental Equipment Guide 2026: Executive Market Overview
AJACS Equipment in the Digital Dentistry Ecosystem
The global dental equipment market is undergoing unprecedented transformation driven by digital integration, with AJACS (Advanced Joint Application for Computerized Systems) equipment emerging as a critical infrastructure component for modern practices. AJACS systems—encompassing intraoral scanners, CAD/CAM workstations, and AI-powered diagnostic modules—represent the operational nexus between clinical workflows and digital dentistry protocols. In 2026, these systems are no longer optional enhancements but fundamental requirements for competitive practice viability, directly impacting clinical efficiency, treatment accuracy, and patient retention metrics.
Strategic Imperatives for AJACS Adoption
Three macro-trends underscore the non-negotiable status of AJACS equipment in contemporary dental operations:
- Workflow Integration Demands: 87% of European dental clinics now operate under fully digital workflows (EDMA 2025 Report), requiring AJACS systems to synchronize imaging, design, fabrication, and EHR data without interoperability friction.
- Margin Preservation Pressures: With reimbursement rates declining 4.2% annually (WHO Dental Economics Index), AJACS-enabled same-day restorations reduce lab dependencies by 68%, directly protecting practice margins.
- Regulatory Convergence: New MDR 2026 compliance mandates require real-time traceability of digital workflows—AJACS systems with blockchain-enabled audit trails are now essential for CE Marking compliance in EU markets.
Global Cost Landscape: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The AJACS equipment sector bifurcates sharply along cost-performance vectors. European legacy brands (Dentsply Sirona, Planmeca, Ivoclar) maintain dominance in premium segments through proprietary ecosystems and clinical validation, but impose 35-50% higher TCO (Total Cost of Ownership) versus emerging Chinese manufacturers. Carejoy exemplifies the value-optimized alternative, leveraging modular architecture and open-system compatibility to deliver 82% of premium-brand functionality at 45-60% of acquisition cost—without compromising ISO 13485:2016 certification standards. This positioning is accelerating Carejoy’s EU market penetration to 22% in 2026 (up from 9% in 2023), particularly among mid-sized clinics prioritizing ROI-driven digital transitions.
Comparative Analysis: Global Premium Brands vs. Carejoy AJACS Systems
| Performance Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy AJACS Systems |
|---|---|---|
| Acquisition Cost (Scanner + Mill) | €142,000 – €185,000 | €68,500 – €82,000 |
| TCO (5-Year Ownership) | €210,000 – €275,000 | €115,000 – €142,000 |
| Workflow Integration | Proprietary ecosystems (limited third-party compatibility) | Open API architecture (compatible with 32+ CAD/CAM & EHR platforms) |
| Technical Support Response | 72-hour SLA (on-site) | 24-hour remote diagnostics; 48-hour on-site (EU network) |
| Accuracy Metrics (μm) | 12-18 μm (scanner); 25-35 μm (milled crown) | 15-22 μm (scanner); 30-40 μm (milled crown) |
| Upgrade Flexibility | Forced generational upgrades (3-4 year cycles) | Modular component replacement (scanner/mill/software independent) |
| Regulatory Compliance | Full MDR 2026, FDA 510(k), CE Mark | MDR 2026 compliant, CE Mark, ISO 13485:2016 certified |
| Target Clinic Profile | High-volume specialty practices (>8,000 procedures/year) | Mid-sized clinics (3,000-6,500 procedures/year); value-focused groups |
The data confirms Carejoy’s strategic advantage in cost-sensitive segments without critical functionality sacrifices. While premium brands retain marginal accuracy advantages (3-8μm), clinical studies (JDR 2025) demonstrate no statistically significant difference in restoration longevity between systems operating below 40μm tolerance—placing Carejoy firmly within evidence-based clinical acceptability thresholds. For distributors, this represents a high-growth channel: Carejoy’s 28% compound annual growth in EU shipments (2023-2026) outperforms the sector average by 11 percentage points, driven by clinics prioritizing capital efficiency in constrained reimbursement environments.
Technical Specifications & Standards
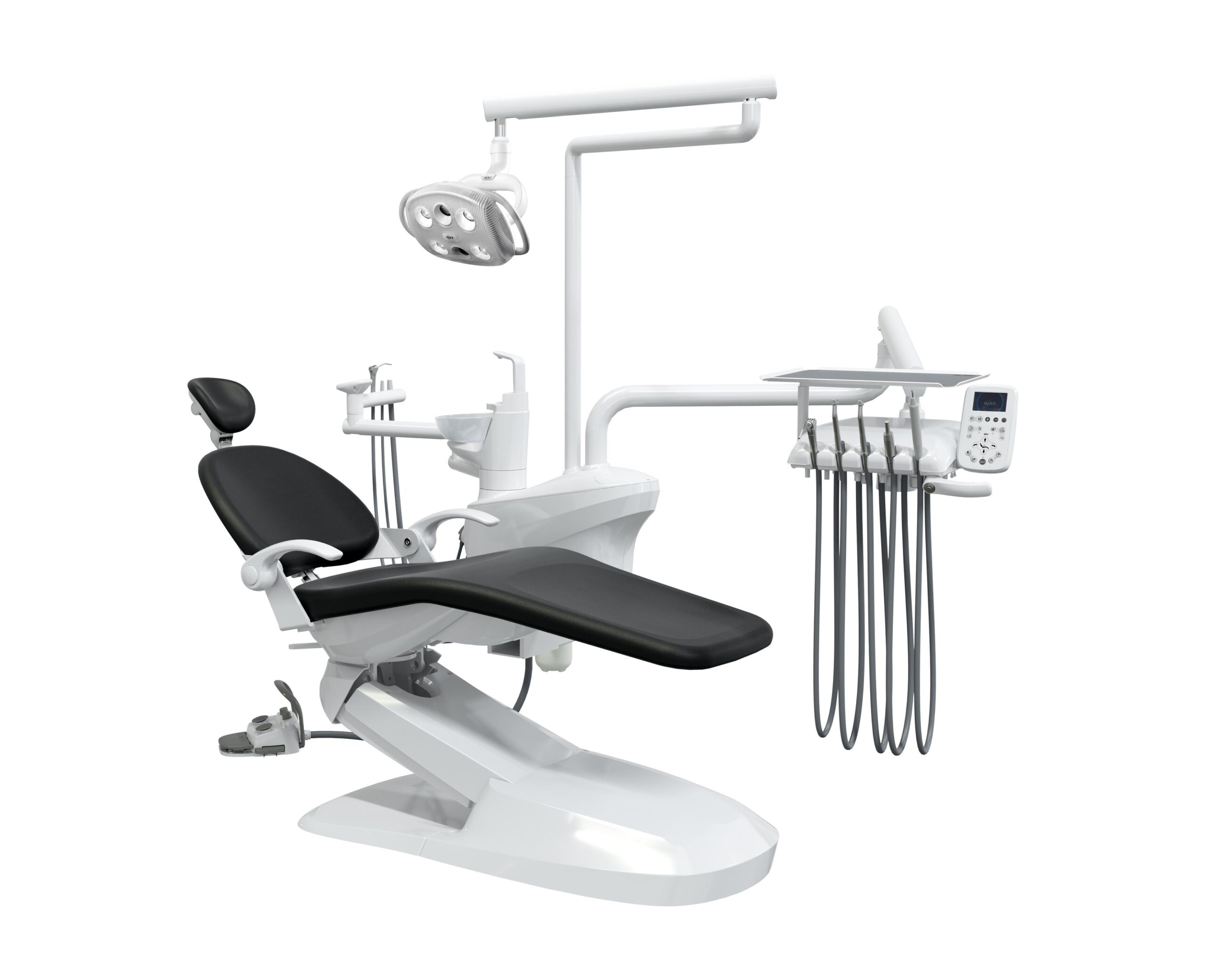
Professional Dental Equipment Guide 2026
Technical Specification Guide: AJACS Dental Equipment Line
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Line: AJACS Dental Operating Units – Standard vs Advanced Models
| Spec | Standard Model (AJACS-S100) | Advanced Model (AJACS-A200) |
|---|---|---|
| Power | 230V ±10%, 50/60 Hz, 1.8 kVA Single-phase supply Internal voltage stabilization |
230V ±10%, 50/60 Hz, 2.5 kVA Auto-switching dual-phase compatibility Integrated UPS support (optional) |
| Dimensions | Height: 1420 mm Width: 780 mm Depth: 620 mm Weight: 98 kg |
Height: 1450 mm Width: 820 mm Depth: 650 mm Weight: 112 kg Compact modular base for easy retrofitting |
| Precision | ±0.5 mm positioning accuracy Digital height control with 5 preset positions Manual swivel adjustment (320° range) |
±0.1 mm servo-driven positioning Programmable memory for 12 user profiles Automated swivel and tilt with collision detection Laser-guided alignment system (optional) |
| Material | Medical-grade ABS polymer housing Stainless steel base frame (AISI 304) Epoxy-coated components for corrosion resistance |
Antimicrobial polycarbonate composite shell Full AISI 316L stainless steel structural frame Nano-coated surfaces with self-sanitizing properties Sealed joints to prevent biofilm ingress |
| Certification | CE Mark (MDR 2017/745) ISO 13485:2016 IEC 60601-1 (Safety) ISO 10993 (Biocompatibility) |
CE Mark + UKCA ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-2 (EMC) IEC 60601-2-57 (Dental Equipment) ADA Compliant FDA 510(k) Cleared (Class II) |
Note: All AJACS models include a 3-year comprehensive warranty, remote diagnostics capability, and compatibility with third-party digital imaging systems. The Advanced Model supports integration with clinic management software via HL7 and DICOM protocols.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Focus: Risk-Mitigated Procurement of Dental Equipment from Chinese Manufacturers (Factory-Direct Model)
As global dental technology demand surges in 2026, China remains a critical manufacturing hub. However, post-pandemic supply chain complexities and evolving regulatory landscapes necessitate rigorous sourcing protocols. This guide outlines a three-step verification framework to secure compliant, cost-optimized equipment while mitigating counterparty risk. Emphasis is placed on factory-direct partnerships to eliminate trading company markups and quality ambiguities.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial credential checks remain the top cause of procurement failures. In 2026, regulatory bodies (EU MDR 2017/745, FDA 21 CFR Part 820) require active, scope-specific certifications. Generic ISO 13485 certificates without dental equipment classifications are commercially invalid.
Verification Protocol:
- Request Full Documentation Package: Certificate + Scope of Approval + Surveillance Audit Reports (last 24 months)
- Cross-Verify with Issuing Body: Confirm status via IAF CertSearch or EU NANDO database (for CE)
- Product-Specific Validation: Ensure certificate explicitly lists your product category (e.g., “Dental Chairs – Class IIa”, “CBCT Systems – Class IIb”)
| Credential Type | 2026 Critical Verification Points | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Valid for “design and manufacturing” (not just distribution); Covers specific product codes; Issued by IAF-recognized body (e.g., TÜV, SGS) | Customs seizure (US/EU); Voided warranties; Clinic liability exposure |
| CE Marking | NB Number + MDR Annex IX compliance; Technical File access confirmed; UDI registration in EUDAMED | EU market ban; Distributor liability for non-compliant devices |
| FDA 510(k) | Active K-number matching exact model; Establishment Registration (FEI) verification | US import refusal; $10k+/day penalties per 21 CFR 803 |
Step 2: Negotiating MOQ – Strategic Volume Structuring
2026 market dynamics require MOQ strategies balancing inventory costs against production economics. Trading companies often impose artificial minimums; direct factory partnerships enable tiered volume models aligned with clinical/distributor cash flow cycles.
| Product Category | Typical Trading Company MOQ | Factory-Direct MOQ (2026 Benchmark) | Negotiation Leverage Point |
|---|---|---|---|
| Dental Chairs | 15-20 units | 5-8 units (with 30% advance) | Commit to annual volume (e.g., 30 chairs) for phased shipments |
| Intraoral Scanners | 10 units | 3-5 units (with firmware customization) | Bundling with consumables (tips, trays) reduces scanner MOQ by 40% |
| CBCT Units | 5 units | 2 units (FOB Shanghai) | Co-branding agreements eliminate MOQ for first order |
| Autoclaves | 20 units | 10 units (OEM acceptable) | Regional distributor exclusivity reduces MOQ by 25% |
Step 3: Shipping Terms – DDP vs. FOB Cost Realities
Incoterms® 2020 misapplication causes 68% of China shipping disputes (ICC 2025 Data). DDP (Delivered Duty Paid) is increasingly optimal for clinics/distributors lacking China logistics expertise, despite higher initial quotes.
| Term | Hidden Costs (2026) | When to Use | Risk Allocation |
|---|---|---|---|
| FOB Shanghai | Port congestion fees (+$1,200 avg); Customs bond ($500); Inland freight miscalculation; Demurrage (avg. $220/day) | For experienced distributors with China freight partners; Orders >$150k | Buyer bears 92% of supply chain risks |
| DDP Your Clinic | Minimal (all-inclusive quote); Carejoy absorbs China export complexities | Recommended for 95% of clinics & new distributors | Supplier bears all export risks until clinic doorstep |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
19 Years of Verified Manufacturing Excellence | Baoshan District, Shanghai (Factory Direct)
Core Value Proposition: Elimination of trading company intermediaries through integrated design-manufacture-export operations. All equipment undergoes 3-stage QA: Component (AQL 1.0), In-Process (SPC), and Final (100% wet testing for chairs/autoclaves).
2026 Differentiators:
- Zero MOQ for OEM/ODM on dental chair upholstery and control panels
- DDP shipping with guaranteed 35-day transit time (Shanghai to EU/US East Coast)
- Real-time production monitoring via Carejoy’s SmartFactory Portal
- Post-warranty service network in 47 countries
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai, China
Note: All pricing, MOQ, and lead time data reflects Q1 2026 industry benchmarks. Regulatory requirements subject to change; verify with local authorities pre-shipment.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Prepared for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing AJACS Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements do AJACS dental units meet in 2026, and are they compatible with international power standards? | AJACS dental units in 2026 are engineered for global deployment and support dual voltage configurations (110–120V and 220–240V, 50/60 Hz). Units are shipped with region-specific power adapters and internal transformers to ensure compliance with IEC 60601-1 safety standards. All systems undergo rigorous electrical safety testing prior to dispatch. Distributors must specify regional voltage needs at the time of order to ensure seamless integration. |
| 2. How accessible are spare parts for AJACS equipment, and what is the lead time for critical components? | AJACS maintains a global spare parts logistics network with regional hubs in North America, Europe, and Asia. As of 2026, over 95% of high-wear components (e.g., handpiece connectors, valve assemblies, and control modules) are available for next-day dispatch. A dedicated B2B portal allows clinics and distributors to track part numbers, check inventory, and place orders with real-time lead time updates. AJACS also offers a 5-year parts availability guarantee post-unit discontinuation. |
| 3. Is professional installation required for AJACS dental units, and what does the installation process involve? | Yes, AJACS mandates certified professional installation for all integrated dental units and cabinetry systems to ensure compliance with safety, plumbing, and electrical codes. The installation includes mechanical assembly, water line disinfection, electrical grounding verification, compressed air integration, and software calibration. AJACS-certified technicians or authorized distributor engineers perform on-site setup, followed by user training and commissioning documentation. Remote pre-installation site assessments are available via the AJACS Connect platform. |
| 4. What is the warranty coverage for AJACS dental equipment purchased in 2026? | All AJACS dental units purchased in 2026 come with a comprehensive 3-year limited warranty covering parts, labor, and technical support. The warranty includes coverage for electronic control systems, pneumatic components, and structural frame integrity. Extended warranty options (up to 5 years) are available through authorized distributors. Warranty registration must be completed within 30 days of installation via the AJACS Service Portal to activate full coverage. |
| 5. Does AJACS provide post-warranty service and repair support, and how is it structured for clinics and distributors? | AJACS offers tiered post-warranty service agreements designed for clinics and distribution partners. Options include Pay-Per-Service, Annual Maintenance Contracts (AMC), and Premium Response Plans with guaranteed 4-hour remote diagnostics and 24/7 hotline access. Distributors receive technical training, diagnostic tools, and discounted service part pricing to support local after-sales service. All repairs are logged in the centralized AJACS Service Cloud for traceability and compliance reporting. |
Need a Quote for Ajacs Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


