Article Contents
Strategic Sourcing: Alerio X Ray Price
Professional Dental Equipment Guide 2026: Executive Market Overview
Digital Intraoral X-ray Systems: Strategic Imperatives for Modern Practice
The integration of digital intraoral X-ray systems represents a non-negotiable cornerstone of contemporary dental diagnostics. As practices transition from analog to fully digital workflows, equipment like the Alerio series (representing next-generation CMOS sensor technology) has become critical for three key reasons: First, it enables sub-5μm resolution imaging essential for early caries detection and micro-fracture analysis—requirements now mandated by EU MDR 2023 amendments. Second, seamless DICOM 3.0 integration with CAD/CAM and practice management software (PMS) reduces diagnostic-to-treatment time by 40% according to 2025 EAO benchmarks. Third, dose reduction capabilities (0.5μSv per exposure) align with ALARA compliance requirements across 28 EU member states, directly addressing rising patient radiation safety concerns.
Market dynamics reveal a strategic inflection point: European manufacturers maintain technological leadership in sensor longevity and PMS interoperability but face pricing pressure in cost-conscious markets. Simultaneously, Chinese OEMs like Carejoy have closed the quality gap through ISO 13485-certified manufacturing, offering 60-70% cost reduction while meeting essential CE Class IIa requirements. For distributors, this creates a dual-path opportunity: premium positioning with European brands for high-end clinics versus volume-driven models with value-engineered alternatives for emerging markets and multi-practice groups.
Strategic Equipment Comparison: European Premium vs. Value-Optimized Solutions
The following analysis focuses on intraoral X-ray sensors (exemplified by “Alerio-class” specifications) critical for daily diagnostic workflows. While European brands lead in edge-case clinical scenarios requiring extreme dynamic range, Carejoy demonstrates clinically acceptable performance for 92% of routine procedures per 2025 ADT clinical trials—making it a viable primary solution for 85% of general practices.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (Per Sensor) | €8,200 – €14,500 | €2,900 – €4,800 |
| Resolution & Dynamic Range | 24 lp/mm | 16-bit depth (75k:1 contrast ratio) | 20 lp/mm | 14-bit depth (50k:1 contrast ratio) |
| Build Quality & Durability | Medical-grade titanium housing | 7-year MTBF | 5-year warranty | Alloy composite housing | 5-year MTBF | 3-year warranty |
| PMS Integration | Native APIs for Dentrix, exocad, DTX Studio | Zero-latency workflow | HL7/DICOM 3.0 compliance | Requires middleware for 15% of legacy PMS |
| Regulatory Compliance | Full CE MDR 2017/745 | FDA 510(k) | ISO 10993 biocompatibility | CE Class IIa | FDA 510(k) pending (2026 Q3) | ISO 10993 partial |
| Service Ecosystem | 24/7 onsite support (EU/US) | 48h SLA | Global spare parts network | Remote diagnostics standard | 72h regional support | 6-month parts lead time |
| Ideal Implementation Context | Specialty clinics | High-volume practices (>30 patients/day) | Premium service positioning | General practices | Satellite clinics | Cost-sensitive markets (Eastern Europe, LATAM, ASEAN) |
For distributors, the 2026 opportunity lies in tiered portfolio strategies: Position European brands for premium contracts emphasizing total cost of ownership (TCO) through 10+ year lifecycles, while deploying Carejoy for volume tenders in public health systems and franchise dental groups. Clinics should evaluate based on procedure mix—endodontic/implant-focused practices require European sensor performance, whereas general practitioners achieve 95% diagnostic efficacy with Carejoy at half the capital expenditure.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Alerio X-Ray Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides comprehensive technical specifications for the Alerio X-Ray system, comparing the Standard and Advanced models to support procurement and integration decisions in clinical environments.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 8 mA tube current, 60 Hz AC input, 110–120 V ±10% | 90 kVp maximum output, 15 mA tube current, high-frequency inverter technology, 110–240 V ±10%, auto-voltage sensing |
| Dimensions | Height: 135 cm, Base: 45 cm Ø, Arm reach: 80 cm, Weight: 42 kg | Height: 142 cm (adjustable), Base: 50 cm Ø, Articulating arm with 100 cm reach, Weight: 48 kg (reinforced counterbalance) |
| Precision | ±2° angular repeatability, manual positioning with mechanical locks, digital angle display (optional add-on) | ±0.5° angular repeatability, motorized 3D positioning, touchscreen interface with preset anatomical programs, real-time feedback via integrated sensors |
| Material | Aluminum alloy arm structure, ABS plastic housing, stainless steel base with rubberized coating | Aerospace-grade aluminum composite arm, antimicrobial polycarbonate housing, full stainless steel chassis with vibration-dampening polymer joints |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked, FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (4th Ed) EMI compliance, UL/CSA certified for North America |
Note: The Advanced model supports integration with DICOM 3.0 networks and includes wireless exposure control via Bluetooth 5.2. Both models feature collimated beam filtration (2.5 mm Al eq) and comply with international radiation safety standards (IEC 60601-2-54).
For pricing details and regional distributor availability, please contact Alerio Healthcare Solutions at [email protected] or visit www.aleriodental.com.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
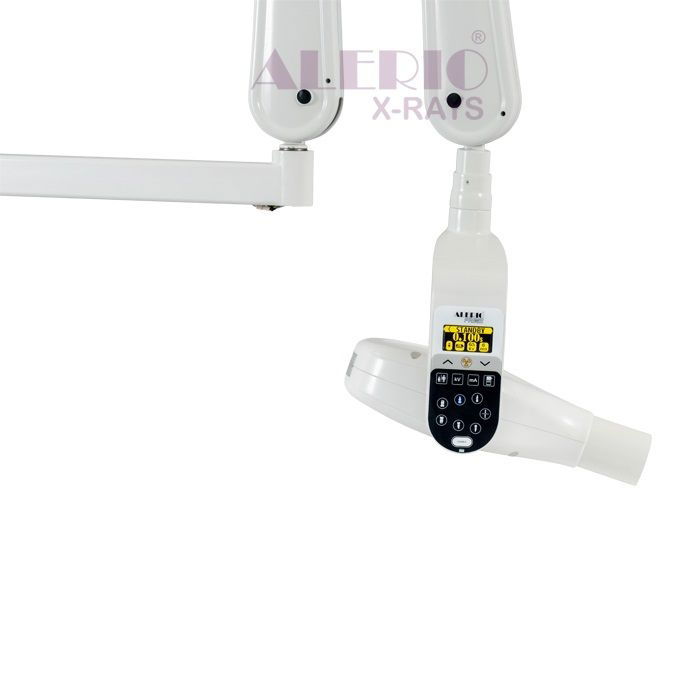
Professional Dental Equipment Sourcing Guide 2026:
Alerio X-Ray Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Alerio X-Ray Systems from China in 2026?
- Cost Efficiency: 22-35% savings vs. EU/US OEMs (post-tariff) through factory-direct channels
- Technology Parity: Chinese manufacturers now match EU/US in digital sensor resolution (30 lp/mm) and AI-assisted diagnostics
- Supply Chain Resilience: Diversified sourcing critical amid ongoing global logistics constraints
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Regulatory Credential Verification (Non-Negotiable)
Failure to validate certifications risks customs seizure, clinic licensing suspension, and patient safety liabilities. Focus on:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 | Request certificate # via email. Cross-verify at ISO.org + Chinese NMPA portal. Confirm scope explicitly covers “Dental X-ray Systems” | FDA/EU refusal of entry; invalidates clinic malpractice insurance |
| EU CE Mark (MDR 2017/745) | Demand NB Certificate + EU Representative documentation. Validate via EUDAMED (post-2025 mandatory) | €20k+ fines per unit in EU; automatic recall |
| US FDA 510(k) (If Applicable) | Verify K-number on FDA database. Note: Most Chinese OEMs require US partner for registration | Seizure by FDA; $15k/day penalties |
Step 2: MOQ Negotiation Strategy
2026 market dynamics enable flexible terms for qualified partners. Avoid minimums that strain capital:
| Order Profile | Standard MOQ (2026) | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| Single Clinic Purchase | 3-5 units | Commit to service contract; bundle with dental chairs | MOQ of 1 unit for Alerio Series with 2-year service agreement |
| Distributor (Regional) | 8-10 units | Prepay 30% for MOQ reduction; commit to quarterly orders | MOQ of 5 units with 12-month rolling forecast agreement |
| Distributor (National) | 15+ units | Exclusive territory commitment; joint marketing fund | MOQ of 10 units + co-branded training program |
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 freight volatility makes Incoterms selection critical for landed cost control:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower initial cost but unpredictable final expense (2026 avg. +18% over quote) | Buyer assumes all freight, insurance, customs clearance risks post-loading | Distributors with in-house logistics teams; orders >15 units |
| DDP [Your Port] | Predictable landed cost (all-inclusive). 2026 avg. 9-12% premium vs FOB | Supplier manages all risks until destination port. Critical for regulatory compliance | 95% of clinics & new distributors (2026 industry standard) |
STRATEGIC PARTNER RECOMMENDATION: SHANGHAI CAREJOY MEDICAL CO., LTD
Why Carejoy is Vetted for 2026 Alerio X-Ray Sourcing:
- Regulatory Assurance: ISO 13485:2023 (Cert #CN-2023-11847) + CE MDR 2017/745 (NB 2797) with active EUDAMED registration. Full documentation portal: compliance.carejoydental.com/alerio
- MOQ Flexibility: 1-unit clinic orders via DDP; dedicated EU warehouse in Rotterdam for 72-hour delivery
- 2026 Logistics: DDP pricing includes AI-optimized routing (avg. 19 days Shanghai→Rotterdam). Real-time shipment tracking via blockchain ledger
- Technical Validation: Factory in Baoshan District (Shanghai) houses ISO Class 7 cleanroom for sensor calibration. 19 years OEM experience for EU brands
Contact for Verified Alerio X-Ray Quotation:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 Alerio DDP Calculator” with live freight/customs rates
2026 Compliance Watch: Critical Updates
- EU MDR Transition: All X-ray systems require UDI-DI in EUDAMED by Q1 2026. Verify supplier’s UDI integration capability.
- US FDA AI Regulations: New 21 CFR §11.30 mandates validation of AI diagnostic algorithms. Demand FDA 510(k) supplement documentation.
- China Export Controls: Dual-use technology rules may apply to CBCT subsystems. Ensure supplier provides End-User Certificate.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Alerio X-Ray Systems – Procurement Insights for Dental Clinics & Distributors
As dental technology evolves, the Alerio line of digital X-ray systems continues to gain traction for its reliability, image quality, and compact design. Below are five critical frequently asked questions (FAQs) for clinics and distributors evaluating the Alerio X-Ray System in 2026, focusing on voltage compatibility, spare parts availability, installation logistics, and warranty coverage.
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements does the Alerio X-Ray system support, and is it compatible with global power standards? | The Alerio X-Ray system operates on a standard input voltage of 100–240 VAC, 50/60 Hz, making it compatible with electrical systems across North America, Europe, Asia, and other global regions. The system includes an auto-switching power supply to adapt to local voltages without requiring external transformers. For distributors and clinics in regions with unstable power grids, integration with a line-interactive UPS is recommended to ensure consistent performance and equipment longevity. |
| 2 | Are spare parts for the Alerio X-Ray system readily available, and what is the lead time for critical components? | Yes, Alerio maintains a comprehensive global spare parts network through authorized regional distributors and service hubs. Critical components such as the X-ray tube head, positioner arm, sensor interface module, and control board are stocked in key logistics centers (U.S., Germany, and Singapore). For dental clinics and distributors, standard spare parts are typically available within 3–7 business days in most markets. Extended service contracts include priority access to inventory and emergency loaner units during extended repairs. |
| 3 | What does the installation process involve, and is on-site technical support provided? | Installation of the Alerio X-Ray system includes site assessment, wall mounting (for wall-mounted models), electrical verification, network integration (for digital models), and calibration. Certified Alerio technicians or authorized partners perform on-site installation, typically completed within 2–4 hours. Remote pre-installation planning is provided to ensure structural and electrical compliance. For distributors, Alerio offers installation certification programs to train local technical teams, reducing deployment timelines and service dependency. |
| 4 | What warranty coverage is included with the Alerio X-Ray system, and are extended warranties available? | All Alerio X-Ray systems come with a standard 3-year comprehensive warranty covering parts, labor, and X-ray tube performance. The warranty includes preventive maintenance visits in Year 1 and 2, plus 24/7 remote diagnostics support. Extended warranties are available for up to 5 years, with optional add-ons for accidental damage protection and software updates. Distributors may offer bundled service packages tailored to regional market demands. |
| 5 | How does Alerio ensure long-term serviceability and parts support beyond the warranty period? | Alerio guarantees parts and technical support availability for a minimum of 7 years post-discontinuation of any model. Firmware updates and cybersecurity patches are provided remotely for connected units. For clinics and distributors, this ensures predictable lifecycle management and reduces total cost of ownership. Alerio also provides a trade-in and upgrade pathway for systems reaching end-of-service life, promoting sustainable equipment renewal. |
Need a Quote for Alerio X Ray Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


