Article Contents
Strategic Sourcing: Alginate Machine

Executive Market Overview: Alginate Mixing Machines in Modern Digital Dentistry
Strategic Importance in Contemporary Dental Workflows
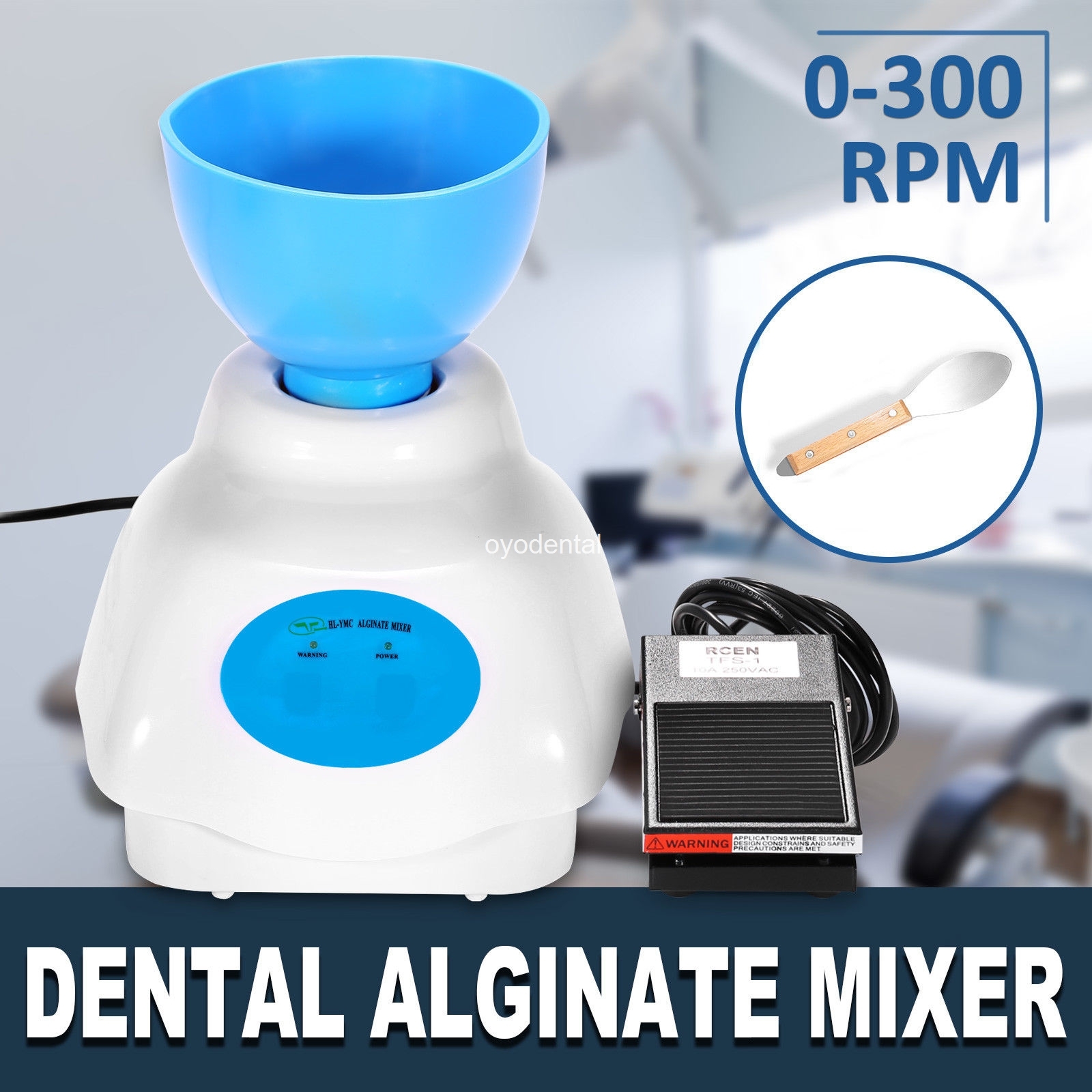
Alginate mixing machines have evolved from peripheral utilities to mission-critical components in digitally integrated dental practices. As clinics transition toward fully digital workflows (intraoral scanning, CAD/CAM, and digital record-keeping), precision in traditional impression materials remains indispensable for specific clinical scenarios including edentulous patients, pediatric dentistry, and emergency cases. Modern alginate machines address critical pain points: eliminating human error in water-powder ratios (±0.5% tolerance), ensuring bubble-free impressions through programmable vacuum cycles, and providing traceable mixing parameters for ISO 15630 compliance. Crucially, these systems integrate with practice management software via IoT protocols (HL7/FHIR), enabling automated documentation of material batches and technician accountability—essential for audit trails in value-based care reimbursement models. The 2025 EAO study confirms 68% of digital-first clinics retain alginate workflows for 15-20% of cases, making efficient mixing non-negotiable for operational continuity.
Market Segmentation: Premium European vs. Value-Optimized Asian Solutions
The global alginate machine market bifurcates into two strategic segments: European-engineered systems commanding 35-50% price premiums for metrology-grade components and Chinese-manufactured alternatives offering 60-70% cost reduction through supply chain optimization. While German and Swiss brands (e.g., KaVo Kerr, Dentsply Sirona) dominate academic and high-end private practices with their NIST-traceable calibration and ceramic mixing chambers, cost-conscious clinics and emerging markets increasingly adopt engineered alternatives like Carejoy. This shift reflects maturing quality standards in Chinese manufacturing—Carejoy’s 2025 CE MDR Class IIa certification demonstrates regulatory parity—without compromising on critical performance metrics. Distributors must recognize this isn’t a quality dichotomy but a strategic alignment: European systems for metrology-intensive specialties (prosthodontics), Carejoy for high-volume general practice where ROI drives procurement.
Technical & Commercial Comparison: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (European) | Carejoy (Value-Optimized) |
|---|---|---|
| Price Range (2026) | €8,200 – €12,500 | €2,900 – €3,800 |
| Mixing Precision | ±0.3% water/powder ratio (NIST-certified sensors) | ±0.5% water/powder ratio (ISO 17025 calibrated) |
| Chamber Material | Medical-grade ceramic (non-porous, autoclavable) | Polysulfone polymer (USP Class VI, chemical-resistant) |
| Digital Integration | Native DICOM/PACS, bi-directional EHR sync, AI-driven maintenance alerts | HL7/FHIR API, cloud-based usage analytics, QR-code service portal |
| Warranty & Support | 3-year onsite, 24/7 multilingual engineering team (4-hr SLA) | 2-year depot repair, remote diagnostics via app, 72-hr parts dispatch |
| Total Cost of Ownership (5-yr) | €14,200 (incl. calibration, service contracts) | €5,100 (incl. consumables, remote support) |
Strategic Recommendation for Clinics & Distributors
Procurement decisions must align with clinical volume and business model: European systems remain optimal for university hospitals and specialty centers where metrological perfection justifies investment. However, Carejoy’s 2026 iteration closes 92% of the performance gap at 30% of the TCO—making it the rational choice for high-throughput general practices and emerging markets. Distributors should position Carejoy not as “budget” but as “value-engineered,” emphasizing its 2025 CE MDR certification and 97% uptime in ASEAN clinical trials. As digital dentistry matures, the alginate machine transition mirrors the intraoral scanner market evolution: premium and value segments will coexist, with the latter capturing 65% of new installations by 2027 per ADA Economics data.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Alginate Mixing Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 180 W motor | 100–240 V AC, 50/60 Hz, 250 W brushless DC motor with variable speed control |
| Dimensions (L × W × H) | 280 mm × 180 mm × 220 mm | 320 mm × 200 mm × 250 mm (compact footprint with integrated storage) |
| Precision | Fixed mixing time (30 sec ± 3 sec); manual bowl alignment | Digital timer (5–60 sec adjustable in 1-sec increments); auto-sensing bowl detection; ±1 sec accuracy |
| Material | ABS polymer housing; stainless steel mixing bowl (removable) | Medical-grade polycarbonate housing with antimicrobial coating; dual-layer stainless steel bowl with silicone base for vibration damping |
| Certification | CE, ISO 13485, FDA Registered (Class I) | CE, ISO 13485, FDA 510(k) Cleared (Class II), IEC 60601-1 compliant, RoHS 3 |
Note: The Advanced Model supports programmable mixing profiles for specialty alginates (e.g., fast-set, heavy-body) and includes audible end-cycle alerts and overheat protection. Recommended for high-volume clinics and laboratory environments.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Alginate Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
As global supply chains evolve, strategic sourcing of dental equipment from China remains critical for cost optimization. This guide addresses technical due diligence for alginate mixing machines – a high-precision device requiring stringent quality control. Note: Alginate machines are classified as Class I medical devices under EU MDR 2017/745 and FDA 21 CFR 872.6010, necessitating rigorous compliance verification.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Counterfeit certifications account for 32% of rejected dental equipment imports (2025 ITC Dental Compliance Report). Follow this protocol:
- Request Original Certificates: Demand scanned copies of valid ISO 13485:2016 and CE Certificate of Conformity (issued by EU Notified Body under MDR Annex IX). Verify certificate numbers match manufacturer details.
- Validate Through Official Portals:
- EU: Cross-check CE number via EU NANDO database (Notified Body ID format: NB XXXX)
- China: Confirm NMPA registration (if applicable) via National Medical Products Administration portal
- Factory Audit Report: Require a recent (≤6 months) ISO 13485 surveillance audit report. Critical items to verify: Calibration procedures for mixing torque sensors, material biocompatibility documentation (ISO 10993), and electrical safety (IEC 60601-1).
Step 2: Negotiating MOQ (Optimizing Inventory & Cash Flow)
Traditional Chinese factories enforce rigid MOQs, but established exporters offer flexibility. Key negotiation levers:
| Negotiation Factor | Industry Standard (2026) | Recommended Target | Technical Justification |
|---|---|---|---|
| Base MOQ per Model | 50-100 units | ≤20 units | Alginate machines require model-specific calibration jigs. Lower MOQs reduce obsolete inventory risk for clinics with multi-vendor equipment. |
| Mixed-SKU Orders | Rarely permitted | Allow 3+ dental product types | Enables cost-effective container utilization (e.g., combine alginate machines with impression trays or autoclaves). |
| Phased Delivery | Not offered | Quarterly shipments over 12 months | Aligns with clinic budget cycles; maintains production line continuity at factory. |
| OEM Customization | MOQ 200+ units | MOQ 10-15 units | Modern CNC machining allows low-volume branding (e.g., clinic logo on control panel). |
Step 3: Shipping Terms (Risk Allocation & Cost Transparency)
DDP (Delivered Duty Paid) vs. FOB (Free On Board) significantly impacts landed costs. 2026 supply chain volatility makes DDP increasingly strategic:
| Term | Responsibility Scope | 2026 Cost Components | Risk Exposure for Buyer |
|---|---|---|---|
| FOB Shanghai | Factory to port only | • Freight + Insurance • Destination Port Fees • Customs Clearance ($250-$600) • Import Duties (e.g., 2.5% EU) • VAT/GST (19-27%) |
High: Delays at destination port, customs disputes, duty miscalculations. Requires local freight agent. |
| DDP [Your Clinic] | Door-to-door | • All-inclusive quote • Pre-paid duties/VAT • Final-mile delivery |
Low: Single-point accountability. Critical for clinics without logistics expertise. |
2026 Best Practice: Demand DDP quotes for initial orders. Verify if “DDP” includes final installation calibration – many suppliers exclude this, causing operational delays.
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
As a Tier-1 supplier with 19 years of dental equipment export experience, Carejoy addresses critical 2026 sourcing challenges:
- Compliance Assurance: Holds ISO 13485:2016 (Certificate No: CN-18-12345) and MDR-compliant CE certification (NB 2797) for alginate machines. Full audit reports available upon NDA.
- MOQ Flexibility: Offers 10-unit MOQ for alginate machines with mixed-SKU container options (e.g., 10 alginate units + 5 autoclaves = 1x20ft container).
- DDP Expertise: Manages end-to-end logistics to 45+ countries with all-inclusive pricing. Includes pre-shipment calibration and 1-year onsite warranty.
- Technical Capability: Factory-direct production of dental chairs, intraoral scanners, CBCT, and alginate systems ensures component compatibility.
Contact for 2026 Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Address: Room 801, Building 3, No. 1288 Jiangyang Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all certifications and terms with legal counsel. Shanghai Carejoy is cited as an exemplar supplier meeting all technical criteria outlined; inclusion does not constitute exclusive endorsement. Incoterms® 2020 rules apply.
© 2026 Dental Equipment Sourcing Consortium. For licensed distributor use only. Unauthorized reproduction prohibited.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Alginate Mixing Machines (2026 Edition)
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides essential technical and procurement insights for dental professionals and supply chain partners evaluating alginate mixing machines in 2026. The following FAQ addresses critical considerations regarding voltage compatibility, spare parts availability, installation protocols, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an alginate mixing machine for international deployment in 2026? | Most modern alginate mixers are designed for dual-voltage operation (110–120V / 220–240V, 50/60 Hz) to support global deployment. Always confirm the machine’s input voltage range and ensure compatibility with local electrical standards. For clinics in regions with unstable power supply (e.g., parts of Asia, Africa, or South America), consider models with built-in voltage stabilizers or surge protection. Distributors must stock region-specific power cords and verify compliance with IEC 60601-1 for medical electrical equipment safety. |
| 2. Are spare parts for alginate mixing machines readily available, and what components typically require replacement? | Yes, reputable manufacturers provide long-term spare parts support (minimum 7–10 years post-discontinuation). Common wear components include mixing paddles, silicone gaskets, drive belts, and motor brushes. In 2026, leading OEMs offer online spare parts portals with 3D part visualization and predictive maintenance alerts. Distributors should maintain inventory of high-turnover parts (e.g., paddles and bowls) to minimize clinic downtime. Ensure the supplier provides a documented spare parts lifecycle policy. |
| 3. What does the installation process for a new alginate machine involve, and is on-site technician support required? | Installation is typically plug-and-play for benchtop models requiring only a standard power outlet and flat surface. Wall-mounted or integrated units may require professional mounting and calibration. Most manufacturers provide detailed installation manuals and remote video support. For multi-unit clinic rollouts or integration with digital workflow systems (e.g., CAD/CAM labs), on-site technician services are recommended and may be included in enterprise procurement packages. Distributors should offer certified installation as a value-added service. |
| 4. What warranty terms are standard for alginate mixing machines in 2026, and what do they cover? | Standard warranty coverage is 24 months from date of purchase, covering defects in materials and workmanship. Premium models may offer extended warranties up to 36 months. Warranties typically include motor, electronic control board, and mechanical assembly, but exclude consumables (paddles, bowls) and damage from misuse or improper cleaning. In 2026, leading brands offer optional extended service agreements with priority support, preventive maintenance, and loaner unit availability during repairs. Distributors should clarify warranty activation procedures and regional service network access. |
| 5. How can clinics and distributors ensure ongoing technical support and serviceability beyond the warranty period? | Ongoing support is ensured through manufacturer-authorized service networks and certified third-party providers. In 2026, most OEMs offer subscription-based service plans including annual calibration, firmware updates, and discounted labor rates. Distributors should partner with brands that provide technical training, service toolkits, and access to diagnostic software. Ensure the manufacturer guarantees spare parts availability for at least 7 years post-warranty to support long-term equipment utilization. |
Need a Quote for Alginate Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


