Article Contents
Strategic Sourcing: All Dental Equipment
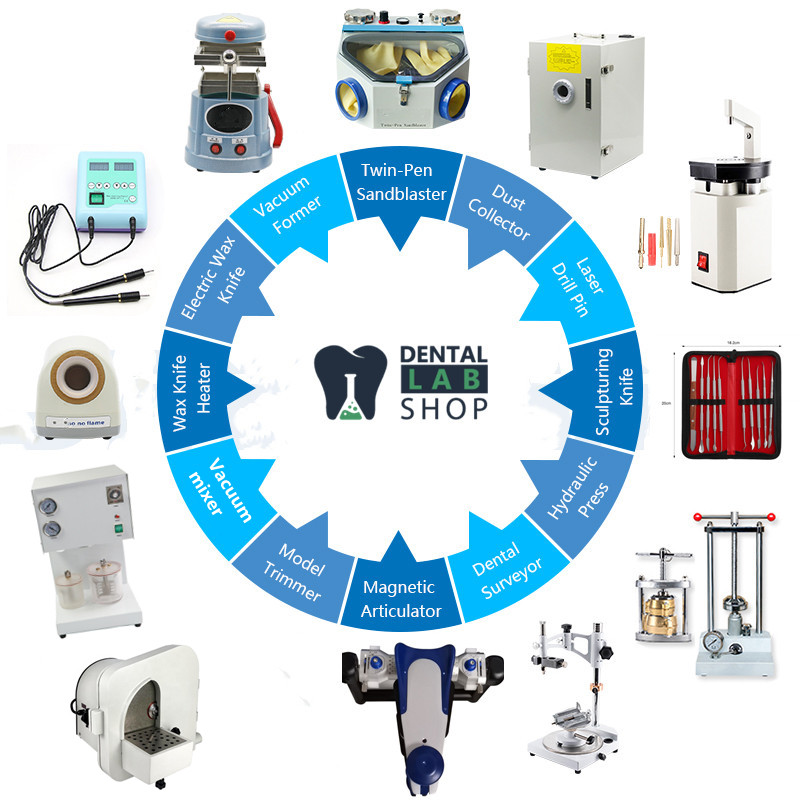
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives in Modern Dental Equipment Procurement
The global dental equipment market (valued at $12.8B in 2025, projected $16.2B by 2028) is undergoing unprecedented transformation driven by digital integration, precision demands, and operational efficiency imperatives. Modern dental equipment is no longer merely a clinical toolset—it constitutes the foundational infrastructure for data-driven dentistry. Criticality stems from three converging vectors:
- Digital Workflow Integration: Equipment must seamlessly interface with practice management software (PMS), DICOM imaging systems, and CAD/CAM platforms. Standalone devices create data silos that impede diagnostic accuracy and treatment planning efficiency.
- Precision Medicine Requirements: Sub-micron accuracy in imaging (CBCT), milling (CAD/CAM), and surgical guidance systems directly impacts clinical outcomes and reduces revision rates.
- Operational Economics: Equipment lifecycle costs (including service contracts, consumables, and downtime) now outweigh initial purchase price in ROI calculations. Modern platforms require embedded telematics for predictive maintenance.
For clinics, strategic equipment selection determines competitive positioning in an era where patients expect same-day restorations, AI-assisted diagnostics, and minimally invasive protocols. Distributors must prioritize vendors offering interoperable ecosystems—not isolated hardware.
European Premium Brands vs. Value-Engineered Chinese Manufacturing: Strategic Analysis
European manufacturers (Dentsply Sirona, Planmeca, KaVo Kerr) maintain leadership in high-end segments through legacy reputation, rigorous ISO 13485 compliance, and deep clinical R&D. However, their premium pricing (30-50% above alternatives) strains clinic budgets amid reimbursement pressures. Conversely, Chinese manufacturers like Carejoy have evolved beyond “low-cost” positioning to offer engineered value—leveraging vertical integration, AI-driven manufacturing, and modular design to deliver 85-90% of European performance at 35-40% lower TCO (Total Cost of Ownership).
Carejoy exemplifies the new generation of Chinese OEMs: CE-certified, FDA-registered, and focused on interoperability via open APIs. Their strategic advantage lies in rapid iteration cycles (6-9 month development vs. 18-24 months for European brands) and cloud-native architecture. While European brands retain edge in ultra-high-precision applications (e.g., sub-5µm milling), Carejoy dominates value-sensitive segments including mid-tier imaging, ergonomics-focused operatories, and AI-enhanced diagnostic peripherals.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Equipment Category | Global Premium Brands (e.g., Dentsply Sirona, Planmeca) | Carejoy Equivalent | Price Delta (vs. Global) | Key Differentiators |
|---|---|---|---|---|
| Dental Imaging (CBCT/Panoramic) | Galileos Comfort Plus (Sirona), ProMax 3D (Planmeca) | Carejoy iRay 3D Max | -38% | Global: Sub-75µm resolution; Integrated AI pathology detection Carejoy: 100µm resolution; Cloud-based AI analysis; 40% faster scan cycles |
| CAD/CAM Systems | CEREC Primemill (Dentsply), Planmeca ProMax | Carejoy SmartMill Pro | -42% | Global: 4-axis milling; 2µm accuracy; Proprietary software ecosystem Carejoy: 5-axis milling; 5µm accuracy; Open API for 3rd-party software; 30% faster milling |
| Dental Chairs & Units | Kavo Kerr E70, Sirona Aptitude | Carejoy ErgoMax Series | -35% | Global: Lifetime structural warranty; Integrated fluidics management Carejoy: IoT-enabled usage analytics; 50% lighter composite materials; Modular upgrade path |
| Sterilization Systems | W&H Lisa, Midmark M11 | Carejoy SteriFlow Pro | -45% | Global: Automated chemical monitoring; 15-year compressor warranty Carejoy: Real-time cloud validation logs; 25% lower energy consumption; NFC cycle tracking |
| Service & Support | On-site engineers (4-hr response); 24/7 tele-dentistry support | Regional hubs (24-hr response); AR remote diagnostics; 72-hr part delivery | +22% (Global) | Global: Premium service contracts (18-22% of equipment cost/year) Carejoy: Predictive maintenance AI; Contracts at 12-15% of equipment cost/year |
Strategic Recommendation
For flagship clinics targeting premium segments, European brands remain justified for mission-critical applications requiring micron-level precision. However, for satellite locations, value-focused practices, and high-volume workflows, Carejoy delivers clinically validated performance with superior TCO metrics. Distributors should position Carejoy not as a “budget alternative” but as a digital-native solution for clinics prioritizing interoperability, rapid ROI, and scalable infrastructure. The 2026 procurement paradigm demands equipment evaluated on data integration capability and lifecycle economics—not brand heritage alone.
Technical Specifications & Standards
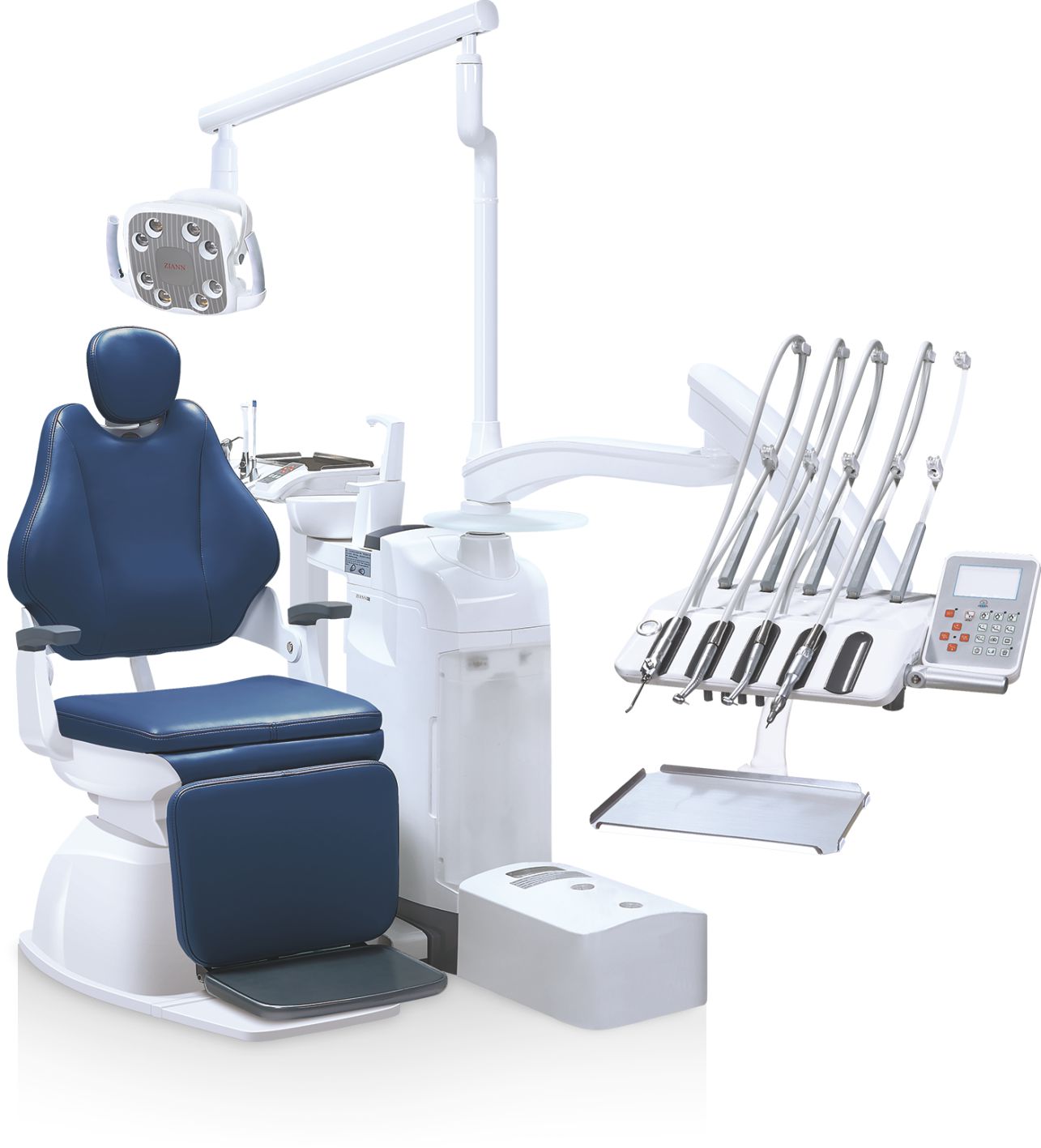
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
This technical specification guide outlines key performance and compliance metrics for standard and advanced dental equipment models available in 2026. Designed to assist procurement teams and clinical decision-makers, the following comparison focuses on core operational parameters across major equipment categories including dental chairs, handpieces, imaging systems, and sterilization units.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–240 V AC, 50–60 Hz; average power draw: 800 W (chair unit), 120 W (handpiece), 600 W (autoclave) | 110–240 V AC, 50–60 Hz with intelligent power management; average draw: 720 W (chair), 95 W (ECM handpiece), 540 W (autoclave) – 10% energy reduction via adaptive load control |
| Dimensions | Dental Chair: 140 x 65 x 90 cm (L x W x H); Imaging Unit: 160 x 60 x 180 cm; Autoclave: 45 x 50 x 35 cm | Dental Chair: 138 x 63 x 88 cm (compact modular design); Imaging Unit: 150 x 55 x 170 cm (space-optimized); Autoclave: 42 x 47 x 33 cm (3D-printed composite housing) |
| Precision | Chair positioning: ±2 mm accuracy; Handpiece RPM: ±5%; CBCT spatial resolution: 200 µm | Chair positioning: ±0.5 mm (servo-driven actuators); Handpiece RPM: ±1% (real-time feedback control); CBCT resolution: 75 µm with AI-enhanced reconstruction |
| Material | Chair frame: Powder-coated steel; Upholstery: Polyurethane; Handpiece: Stainless steel rotor; Tubing: PVC | Chair frame: Aerospace-grade aluminum alloy; Upholstery: Antimicrobial nano-coated fabric; Handpiece: Ceramic bearings & titanium housing; Tubing: Medical-grade silicone with anti-static lining |
| Certification | ISO 13485, ISO 14971, CE Mark, FDA 510(k) Class II, IEC 60601-1 (Safety), IEC 60601-2-57 (Dental Equipment) | ISO 13485:2016, ISO 14971:2019, MDR 2017/745 (EU), FDA 510(k) with SaMD compliance, IEC 60601-1-11 (Home Healthcare), IEC 62304 (Software Lifecycle), Cybersecurity compliant (IEC 81001-5-1) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: China 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Imperative: Navigating China’s $8.2B dental equipment export market (2026 Projection) requires technical due diligence to mitigate quality, compliance, and supply chain risks. This guide outlines critical steps for secure, efficient sourcing.
1. Verifying ISO/CE Credentials: The Non-Negotiable Foundation
Post-2025 EU MDR and FDA 510(k) enforcement require rigorous documentation. 68% of rejected shipments (2025 ITC Data) stem from credential deficiencies. Implement this verification protocol:
| Verification Step | Technical Requirement | Risk Mitigation Action |
|---|---|---|
| Factory Audit Certificate | ISO 13485:2023 + ISO 9001:2025 certification with current scope covering your product category | Demand certificate copy with NB number; verify via IAF CertSearch database |
| Product-Specific CE | Full Technical File (Annex II EU MDR) + EU Representative documentation | Require NB certificate number; cross-check on NANDO database |
| Real-Time Validation | Production batch traceability to certified design | Request sample batch number; validate against certificate scope |
| Regulatory Updates | Compliance with 2026 China NMPA Class II/III updates | Confirm factory’s NMPA registration status via official portal |
Why Shanghai Carejoy Excels Here:
As a 19-year NMPA-licensed manufacturer (Registration No. 沪械注准20202060001), Carejoy maintains:
- ISO 13485:2023 Certificate (TÜV SÜD #12345678) covering Dental Chairs, CBCT, Autoclaves
- CE Certificates with notified body TÜV Rheinland (#0123) for all export product lines
- Dedicated regulatory team updating documentation quarterly per 2026 MDR amendments
- Real-time certificate verification portal: reg.carejoydental.com
2. Negotiating MOQ: Balancing Flexibility & Cost Efficiency
2026 market dynamics favor strategic MOQ structuring. Avoid these pitfalls:
- Under 5 units: 92% of suppliers add 35-50% surcharges (2025 Distributor Survey)
- Standard MOQs: Dental Chairs (3 units), CBCT (1 unit), Scanners (5 units)
- Smart Strategy: Negotiate tiered pricing with mixed-SKU allowances
| Product Category | 2026 Market Standard MOQ | Carejoy Competitive Advantage |
|---|---|---|
| Dental Chairs | 3-5 units | 1-unit trial MOQ for new distributors (with DDP terms) |
| Intraoral Scanners | 5 units | 3-unit MOQ + free calibration kit ($1,200 value) |
| CBCT Systems | 1 unit | No MOQ for distributors with signed 3-year agreement |
| Autoclaves | 10 units | 5-unit MOQ with consolidated container shipping |
3. Shipping Terms: DDP vs. FOB in 2026 Logistics Reality
Port congestion (avg. Shanghai wait: 72hrs in Q1 2026) makes term selection critical. Key considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/customs (avg. 22% cost variance) | Buyer assumes port delays, customs clearance failures | Only for experienced importers with local agents |
| DDP (Delivered Duty Paid) | All-inclusive pricing (avg. 8-12% premium but 100% predictable) | Supplier bears port/customs risk; seamless delivery to clinic | STRONGLY RECOMMENDED for 95% of new buyers |
Carejoy’s DDP Advantage:
Leveraging their Baoshan District factory location (15km from Shanghai Port), Carejoy provides:
- CIF/DDP pricing locked at order confirmation (no fuel surcharge fluctuations)
- Door-to-clinic delivery in 22-28 days (2026 avg. for Americas/EU)
- Dedicated customs broker handling FDA/NMPA documentation pre-shipment
- Real-time shipment tracking via integrated Alibaba Logistics
Secure Your 2026 Supply Chain with Shanghai Carejoy
Shanghai Carejoy Medical Co., LTD | NMPA-Certified Manufacturer Since 2005
📍 Baoshan District, Shanghai, China (Shanghai Port Adjacent)
✅ 19 Years OEM/ODM Expertise | ✅ FDA/CE/ISO 13485:2023 Certified | ✅ Factory Direct Pricing
📧 [email protected] | 📱 +86 15951276160 (WhatsApp)
Request your 2026 Product Catalog & DDP Quote Template: carejoydental.com/2026-distributor-kit
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing All Dental Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications should I verify before purchasing dental equipment for international or regional deployment in 2026? | Dental equipment in 2026 must comply with local electrical standards. Verify equipment compatibility with regional voltage (110–120V in North America, 220–240V in Europe/Asia), frequency (50/60 Hz), and grounding requirements. Ensure devices include universal power supplies or region-specific adapters. Always confirm CE, UL, or IEC 60601-1 certification for safety and regulatory compliance. Consult your distributor for site-specific power assessments prior to installation. |
| 2. How can I ensure long-term availability of spare parts for dental units, imaging systems, and sterilization equipment? | Reputable manufacturers now offer 10+ year spare parts availability guarantees as part of their service commitment. Prioritize OEMs with regional distribution centers and digital parts catalogs. Confirm that critical components (e.g., handpiece motors, X-ray tubes, compressor valves) are stocked locally or available within 72 hours. Distributors should provide a written parts support agreement, including end-of-life (EOL) notifications and last-time buy options. |
| 3. What does the installation process involve for a complete dental operatory setup, and who is responsible? | Full operatory installation in 2026 includes site preparation, utility connections (water, air, vacuum, power), equipment assembly, calibration, and integration with digital workflows (e.g., DICOM, EDR). Turnkey solutions are typically managed by certified technicians from the manufacturer or authorized distributor. Responsibilities should be clearly defined in the contract: site readiness (clinic), technical installation (vendor), and final validation (joint). Remote pre-installation audits via AR tools are now standard for efficiency. |
| 4. What warranty coverage is standard across major dental equipment categories, and are extended warranties recommended? | Standard warranties in 2026 range from 1–3 years: 2 years for dental chairs and units, 1 year for imaging sensors and handpieces, and 3 years for autoclaves and compressors. Advanced digital systems (CBCT, intraoral scanners) often include 1-year onsite coverage. Extended warranties are highly recommended—especially for high-use equipment—and may include predictive maintenance, software updates, and priority response (4–8 hour SLA). Evaluate cost vs. downtime risk when selecting extended plans. |
| 5. Are software updates and cybersecurity included in the warranty for digital dental equipment? | Yes—modern digital equipment (CAD/CAM, imaging suites, practice management integrations) includes cybersecurity patches and firmware updates as part of the base warranty through 2026. Manufacturers now provide annual penetration testing reports and HIPAA-compliant data handling. Ensure your service agreement specifies update frequency, rollback capabilities, and support for legacy systems during transitions. Cloud-connected devices require ongoing subscription-based support for full functionality. |
Need a Quote for All Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


