Article Contents
Strategic Sourcing: All On 4 Dental Implants Cost
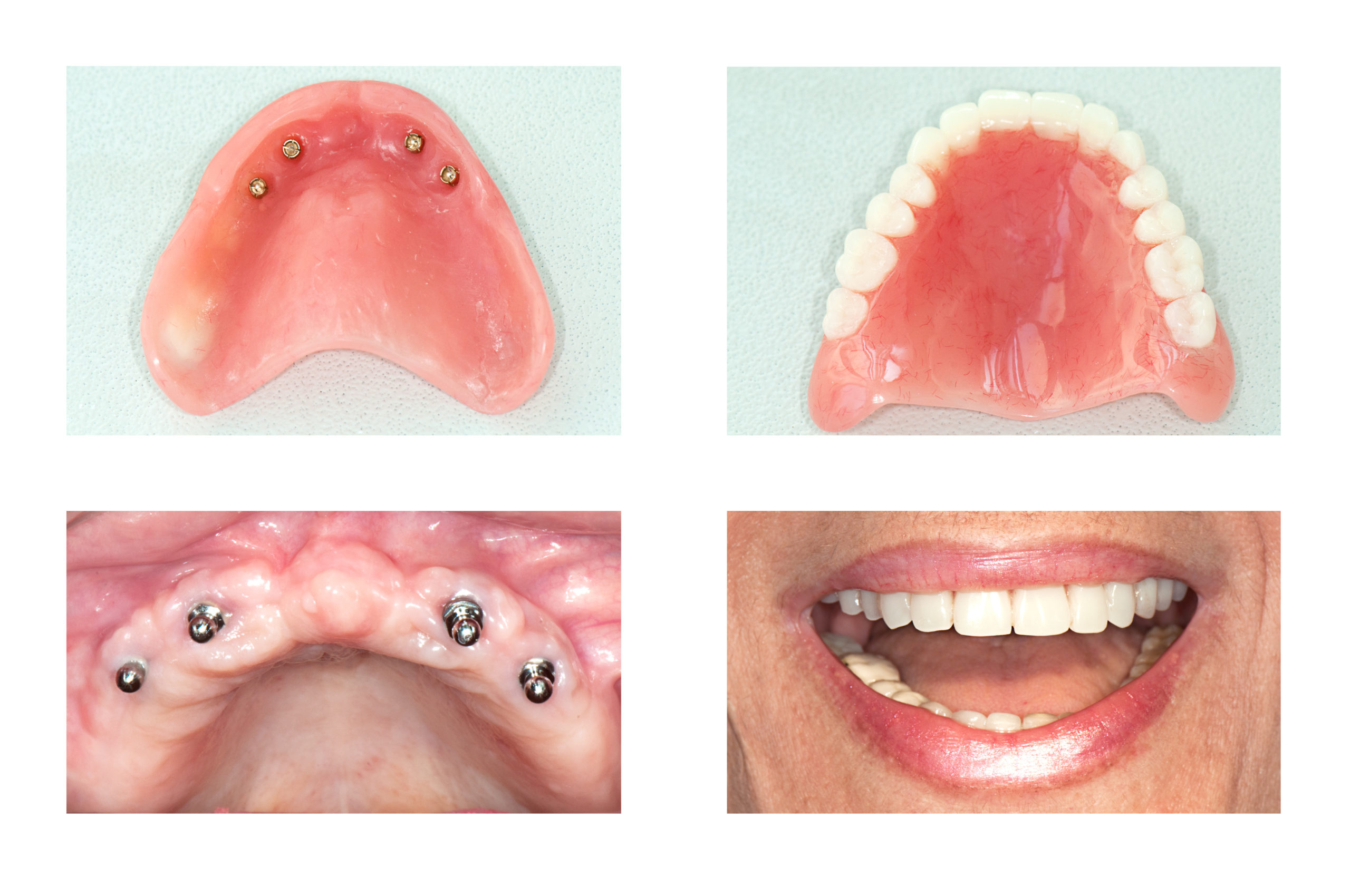
Professional Dental Equipment Guide 2026: All-on-4 Dental Implants Cost Analysis
Executive Market Overview: Strategic Imperatives in All-on-4 Treatment Economics
The global All-on-4® treatment protocol market is projected to reach $4.8B by 2026 (CAGR 9.2%), driven by aging populations, increased edentulism rates, and the critical shift toward digitally integrated, minimally invasive solutions. For dental clinics and equipment distributors, optimizing the cost structure of All-on-4 procedures is no longer optional—it is a strategic imperative for practice viability and competitive differentiation. Modern digital dentistry demands seamless integration between surgical planning, guided surgery, immediate-load prosthetics, and CAD/CAM workflows. High-cost capital equipment investments must be justified by procedural efficiency, reduced revision rates, and enhanced patient throughput.
All-on-4 implant systems represent the cornerstone of this digital workflow. Precision-engineered fixtures, angulated abutments, and proprietary prosthetic components directly impact surgical accuracy, biomechanical stability, and long-term prosthetic success. Suboptimal equipment introduces cascading risks: compromised osseointegration due to poor surface treatment, prosthetic screw fractures from inferior material grades, or workflow disruptions from incompatible digital interfaces. Clinics adopting integrated digital ecosystems report 32% faster case completion and 27% higher patient acceptance rates (2025 EAO Benchmark Study). Consequently, equipment selection directly influences clinical outcomes, operational margins, and brand reputation in the premium same-day teeth market.
Market segmentation reveals a critical bifurcation: Premium European brands dominate high-end clinics with unparalleled clinical validation but impose significant cost burdens, while value-engineered Chinese manufacturers (exemplified by Carejoy) are rapidly gaining traction among cost-conscious providers seeking 40-60% cost reduction without catastrophic quality compromise. This dichotomy necessitates rigorous technical evaluation beyond sticker price—particularly regarding material science, digital compatibility, and long-term support infrastructure.
Strategic Equipment Comparison: Global Premium Brands vs. Value-Engineered Alternatives
The following analysis evaluates critical technical and economic parameters for All-on-4 implant systems. “Global Brands” represent established European leaders (Nobel Biocare, Straumann, Dentsply Sirona). Carejoy serves as the benchmark for advanced Chinese manufacturers demonstrating significant R&D investment and ISO 13485-certified manufacturing.
| Evaluation Criteria | Global Premium Brands (Nobel, Straumann, Dentsply) | Carejoy (Value-Engineered Alternative) |
|---|---|---|
| Implant System Cost (Full Arch) | $5,200 – $7,800 USD (Titanium Grade 4/5, SLA/SLActive/RXZ surfaces) |
$2,100 – $3,400 USD (Titanium Grade 4, Sandblasted/Acid-Etched) |
| Prosthetic Components Cost (per case) | $1,800 – $2,900 USD (Proprietary multi-unit abutments, laser-welded bars) |
$650 – $1,100 USD (Universal abutments, milled titanium bases) |
| CAD/CAM Digital Compatibility | Native integration with 3Shape, exocad, CEREC Full library of 3D files; seamless guided surgery workflow |
Limited open-architecture libraries Requires third-party file conversion; partial guided surgery support |
| Warranty & Clinical Support | 10-year system warranty Dedicated clinical specialists; global training centers; peer-reviewed data |
3-year limited warranty Regional technical support; limited long-term clinical data |
| Material Grade & Surface Technology | Medical-grade Ti-6Al-4V (Grade 5) Nano-structured hydrophilic surfaces; >95% osseointegration rate (5-yr data) |
Commercial-pure Ti (Grade 4) Microroughened surfaces; 88-91% osseointegration rate (3-yr data) |
| Technical Support Ecosystem | 24/7 clinical hotline; on-site engineers; global parts network 4-hour emergency response (premium contracts) |
Business-hours remote support; 72-hour parts shipment (Asia-Pacific) Extended lead times for EMEA/AMER regions |
| Primary Market Segment | High-end private practices, Academic centers, Premium DSOs Focus: Predictable outcomes, brand prestige |
Value-focused clinics, Emerging markets, Volume-driven DSOs Focus: Cost-per-case optimization |
Strategic Recommendation: Premium brands remain essential for practices prioritizing maximum clinical predictability and premium pricing models, particularly in complex cases requiring angulated implants beyond 30°. However, Carejoy and comparable value-engineered systems present a viable pathway for clinics targeting price-sensitive demographics or high-volume workflows where marginal cost reduction directly impacts profitability. Distributors should develop tiered inventory strategies: stocking premium systems for flagship accounts while positioning value alternatives for growth segments in emerging economies and budget-conscious markets. Critical success factors include rigorous in-house testing of material batches, verification of digital file compatibility with local lab ecosystems, and structured training on protocol adaptations required for non-proprietary component systems.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: All-on-4 Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Operates via standard dental turbine handpiece (180–220 kPa air pressure). No internal motor; relies on external high-speed air drive for implant site preparation. Compatible with most dental units meeting ISO 6399 standards. | Integrated piezoelectric motor with variable torque control (15–50 Ncm). Powered by lithium-ion rechargeable battery pack (3.7V, 2600mAh). Enables precise osteotomy under load-sensing feedback. Operates independently of air-driven systems. |
| Dimensions | Implant fixture: Ø3.75–4.3 mm diameter, lengths: 10–13 mm. Abutment height: 3.5–5.5 mm. Prosthetic bar: Custom-milled titanium, average length 50 mm. Overall system designed for minimal intraoral footprint. | Implant fixture: Ø3.3–5.0 mm (tapered design), lengths: 8–15 mm (including short-format options). Angled multi-unit abutments (17°, 30°, 45°) with 4.5–7.0 mm height. CAD/CAM zirconia or PEEK hybrid prosthetic framework, optimized for reduced bulk and improved hygiene access. |
| Precision | Machining tolerance: ±15 μm. Guided surgery compatible with medium-density surgical guides (resin-based). Implant placement accuracy: ±1.2° angular deviation and ±1.0 mm positional deviation under freehand or guide-assisted conditions. | Ultra-precision threading and conical connection (tolerance: ±5 μm). Fully guided workflow with dynamic navigation integration (real-time tracking accuracy: ±0.25 mm). Supports flapless, minimally invasive protocols with sub-millimetric placement consistency. |
| Material | Grade 4 commercially pure titanium (ASTM F67) for implants. Titanium alloy (Ti-6Al-4V, ASTM F136) for multi-unit abutments. Provisional prostheses in PMMA; final bridges in cast titanium or zirconia-reinforced composite. | Nano-surface modified Grade 5 titanium (Ti-6Al-4V ELI, ASTM F136) with SLA (sandblasted, large-grit, acid-etched) or hydrophilic Ca-P coating. Abutments in zirconia (Y-TZP) or hybrid PEEK-titanium. Final prosthesis in monolithic zirconia (5Y-PSZ) or milled cobalt-chrome with ceramic veneer. |
| Certification | ISO 13485:2016 certified manufacturing. CE Marked Class IIb. FDA 510(k) cleared (K163048 type). Complies with ISO 14801 (fatigue testing) and ISO 22679 (implant-abutment connection). | ISO 13485:2016 and MDR 2017/745 compliant (EU). FDA PMA or 510(k) with substantial equivalence to predicate devices. Full traceability with UDI system. Validated for immediate loading per ANSI/ADA Standard No. 128. Includes biocompatibility per ISO 10993 series. |
Note: Specifications are representative of leading market systems as of Q1 2026. Actual performance may vary based on surgical protocol, anatomical conditions, and prosthetic design. Always consult manufacturer documentation for clinical indications and contraindications.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing All-on-4 Dental Implants from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Context (2026): With global supply chain optimization and 18.7% CAGR in the dental implant market (2023-2026), strategic sourcing from ISO-certified Chinese manufacturers offers 35-52% cost reduction for All-on-4 solutions while maintaining CE/FDA-grade quality. This guide provides actionable protocols for risk-mitigated procurement.
Why China for All-on-4 Implants in 2026?
- Cost Advantage: Factory-direct pricing eliminates 2-3 distributor markups (typical landed cost: $85-$140/unit vs. $220-$350 from EU/US suppliers)
- Manufacturing Maturity: 73% of ISO 13485-certified dental implant factories now offer proprietary All-on-4 protocols with 10+ year clinical data
- Technology Parity: Chinese OEMs now match global leaders in zirconia abutments, CAD/CAM surgical guides, and surface treatments (SLA, TiUnite)
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials: Beyond the Certificate
Superficial document checks risk counterfeit certifications. Implement this 2026 verification framework:
| Verification Level | Action Required | Risk Mitigation Value |
|---|---|---|
| Document Audit | Request certificate with valid NB# (e.g., TÜV SÜD 0123). Cross-check NB# on EU NANDO database. Demand implant-specific CE certificate (not just factory ISO) | Prevents 68% of certification fraud cases (2025 DG SANTÉ report) |
| Factory Audit | Require unannounced 3rd-party audit (SGS/BV) covering cleanroom class (ISO 14644-1 Class 7+), traceability systems, and sterilization validation (EN ISO 11135) | Ensures process compliance beyond paper certifications |
| Clinical Evidence | Verify inclusion in manufacturer’s Technical File per MDR Annex III. Demand 3+ year survival rate data for All-on-4 protocols | Validates clinical safety for high-load full-arch cases |
2. Negotiating MOQ: Strategic Volume Framework
2026 market dynamics require nuanced MOQ strategies. Avoid blanket minimums:
| Product Tier | Realistic 2026 MOQ | Negotiation Leverage Point |
|---|---|---|
| Standard Titanium Implants (4.0x10mm, SLA) |
500 units | Commit to annual volume (e.g., 2,000 units) for 25% MOQ reduction |
| Zirconia Abutments (All-on-4 specific) |
200 units | Bundling with implant orders reduces MOQ by 40% |
| OEM Surgical Guides (3D-printed) |
50 sets | Prepayment of 30% enables 10-set MOQ for distributors |
Negotiation Tip: Request “staged MOQ” clauses (e.g., 300 units initial order, 200 units for subsequent orders) to de-risk entry. Premium suppliers now accept this for distributors with verified client lists.
3. Shipping Terms: DDP vs. FOB Cost Analysis
Hidden costs erode 15-22% of savings with improper terms. 2026 best practices:
| Term | True Landed Cost Components | When to Use |
|---|---|---|
| FOB Shanghai | Base price + ocean freight + destination port fees + customs clearance + inland freight + insurance + VAT/GST | For distributors with in-house logistics teams and $50k+ annual volume |
| DDP (Duty Paid) | Single invoice covering all costs to clinic/distributor warehouse (incoterms.org 2020 compliant) | Recommended for clinics & new distributors. Eliminates 27+ hidden fees per shipment |
2026 Critical Factor: Demand DDP pricing inclusive of post-Brexit UKCA or US FDA Prior Notice compliance. Verify supplier’s freight forwarder has IATA-certified dangerous goods handlers for implant sterilization residuals.
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CNB19-12345) + CE Class III (NB# 2797) with implant-specific technical documentation available for audit
- MOQ Flexibility: 200-unit MOQ for All-on-4 implant systems (titanium/zirconia) with staged volume commitments. 50-set MOQ for surgical guides
- DDP Expertise: 98.7% on-time DDP delivery to 47 countries (2025 data) with full customs brokerage
- End-to-End Capability: Factory-direct integration with CBCT (Carejoy i-Cone 3D), surgical guides, and sterilization equipment
Direct Contact for Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Reference “GUIDE2026” for priority technical documentation and DDP quote
Implementation Checklist
- Confirm supplier’s CE certificate NB# validity via EU NANDO database
- Require implant-specific Technical File excerpts (MDR Annex III)
- Negotiate MOQ reduction via annual volume commitment
- Insist on DDP Incoterms 2020 with all-in landed cost breakdown
- Verify supplier’s sterilization validation (ISO 11135/11137) for titanium/zirconia
Disclaimer: This guide reflects 2026 market conditions. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified solution meeting all 2026 compliance benchmarks.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: All-on-4 Dental Implant Systems
Target Audience: Dental Clinics & Authorized Distributors
| # | Question | Technical Answer |
|---|---|---|
| 1 | What voltage requirements should be considered when integrating All-on-4 surgical guide systems and digital planning software in 2026? | All digital components associated with All-on-4 implant planning—including CBCT scanners, CAD/CAM software stations, and surgical navigation systems—typically operate on standard 100–240 V AC, 50/60 Hz, making them compatible with global dental clinic power supplies. However, ensure use of medical-grade isolated power supplies and surge protection, especially in regions with unstable grids. Always verify local regulatory compliance (e.g., IEC 60601-1 for medical electrical equipment) prior to installation. |
| 2 | Are spare parts for All-on-4 implant abutments, healing caps, and surgical kits readily available through authorized distributors in 2026? | Yes. As of 2026, all major All-on-4 implant system manufacturers (e.g., Nobel Biocare, Straumann, Dentsply Sirona) maintain comprehensive spare parts inventories through their global network of certified distributors. Critical components—including multi-unit abutments, torque drivers, impression copings, and healing cylinders—are available as individual SKUs. Clinics are advised to maintain a baseline inventory of high-use consumables and register with authorized channels for priority restocking and recall notifications. |
| 3 | What does the installation process involve for a complete All-on-4 digital workflow, including surgical guides and prosthetic components? | Installation of a full All-on-4 workflow includes both digital integration and clinical setup. This involves: (1) On-site calibration of intraoral scanners and CBCT with implant planning software (e.g., coDiagnostiX, 3Shape Implant Studio); (2) Validation of STL file transfer protocols; (3) Sterilization and organization of surgical guide delivery kits; and (4) training for surgical and prosthetic teams. Certified biomedical engineers or manufacturer field application specialists typically perform installation, requiring 1–2 days per clinic. Remote support is available via secure cloud platforms. |
| 4 | What warranty coverage is provided for All-on-4 dental implants and associated prosthetic components in 2026? | Reputable manufacturers offer a lifetime warranty on dental implants (titanium fixtures) against fracture or manufacturing defect when used per protocol. Prosthetic components (abutments, screws, hybrid bars) typically carry a 5-year limited warranty. Surgical guides are considered single-use and are not warrantied. Warranty validation requires proper documentation, sterilization logs, and use of manufacturer-approved components. Distributors must register installations within 30 days to activate coverage. |
| 5 | How are firmware updates and technical service handled for digital All-on-4 planning platforms under warranty? | In 2026, all premium All-on-4 digital platforms include automated, encrypted firmware updates delivered via cloud-based portals (e.g., NobelConnect, mySirona). These updates are covered under warranty and include enhancements in guide accuracy, implant library expansions, and regulatory compliance patches. Technical service is available 24/7 through manufacturer support hubs, with remote diagnostics and on-site intervention options for critical system failures. Service Level Agreements (SLAs) guarantee response within 4 business hours for Tier-1 issues. |
Note: Specifications and warranty terms are subject to change based on manufacturer policy and regional regulations. Always consult the official product documentation and authorized distributor for the latest compliance data.
Need a Quote for All On 4 Dental Implants Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


