Article Contents
Strategic Sourcing: All On 4 Dental Implants Price

Professional Dental Equipment Guide 2026: Executive Market Overview
All-On-4® Dental Implant Systems: Strategic Pricing & Value Analysis
Prepared Exclusively for Dental Clinic Decision-Makers & Global Distributors
Market Imperative: The Digital All-On-4® Revolution
The All-On-4® protocol represents a cornerstone of modern digital dentistry, directly addressing the accelerating global demand for immediate-load full-arch rehabilitation. With edentulism affecting 30+ million patients in OECD nations alone and digital workflow adoption exceeding 78% in premium clinics (2025 EAO Report), this solution is no longer elective—it’s clinically and economically essential. Integrated digital workflows (CBCT → surgical guide → CAD/CAM prosthesis) reduce treatment time by 60%, minimize surgical complications through guided protocols, and deliver predictable biomechanical outcomes. Critically, All-On-4® systems must now seamlessly interface with intraoral scanners, AI-driven treatment planning software, and chairside milling ecosystems. Clinics lacking this integrated capability face declining case acceptance rates and competitive obsolescence in the $5.2B global immediate-load implant market (2026 Projection: Grand View Research).
Strategic Value Proposition: Beyond Component Cost
Pricing analysis must extend beyond per-implant cost. The true value equation encompasses:
- Digital Ecosystem Integration: Compatibility with open-architecture software (e.g., exocad, 3Shape) avoiding vendor lock-in
- Prosthetic Flexibility: Universal abutment design supporting PMMA, zirconia, and hybrid frameworks
- Guided Surgery Precision: Tolerance levels ≤50μm for drill guides ensuring optimal primary stability
- Service Velocity: Critical downtime reduction via 48-hour component replacement SLAs
Clinics prioritizing ROI must evaluate total cost of ownership (TCO), where premium brands command 35-45% higher initial investment but offer marginally faster case completion in complex scenarios. Value-engineered systems now deliver 92%+ clinical equivalence at significantly lower TCO—a paradigm shift reshaping procurement strategies.
Global Competitive Landscape: Premium vs. Value-Engineered Systems
The European premium segment (Nobel Biocare, Straumann, Dentsply Sirona) maintains dominance in high-complexity referrals through legacy clinical data and brand trust. However, stringent reimbursement pressures in EU markets and distributor margin compression are accelerating adoption of ISO 13485-certified alternatives. Chinese manufacturers, particularly Carejoy, have transitioned from “low-cost” to “value-engineered” players through strategic R&D investments in digital compatibility and biocompatible materials. Carejoy’s 2025 launch of the ProArch Digital Suite—featuring ANSI/ADA-compliant Ti-6Al-4V Grade 5 implants with 0.7mm platform switching and universal prosthetic interfaces—demonstrates technical parity in 85% of routine cases while reducing system costs by 30-40%.
| Category | Global Premium Brands (Nobel, Straumann, Dentsply Sirona) |
Carejoy |
|---|---|---|
| Implant System Cost (Full Arch) | $8,500 – $12,200 USD | $5,100 – $7,400 USD |
| Prosthetic Component Cost (Abutments/Temporaries) | $1,800 – $2,600 USD | $950 – $1,350 USD |
| Guided Surgery Compatibility | Proprietary software only (vendor lock-in); 0.3° angular deviation | Open API for 3Shape/exocad; 0.4° angular deviation (ISO 16409 compliant) |
| Clinical Documentation & Longevity Data | 15+ year survival data (95.2%); 500+ peer-reviewed studies | 7-year survival data (93.8%); 85+ clinical validations; ISO 14801 fatigue tested |
| Service Network Coverage | Global 24/7 support; 4-hour emergency response (EU/US); 98% part availability | EU/US hubs (Berlin, Miami); 48-hour critical component SLA; 90% part availability |
| Target Clinic Profile | High-volume referral centers; complex cases; insurance-preferred networks | Mid-tier private practices; value-focused DSOs; emerging markets; high-volume routine cases |
Strategic Recommendation for Distributors & Clinics
Clinics: Implement a tiered procurement strategy—reserve premium systems for complex anatomical cases (>30° cantilever) while deploying value-engineered solutions like Carejoy for routine protocols. This optimizes TCO without compromising clinical outcomes, with potential savings of $18,000+ per annual case volume of 20.
Distributors: Position Carejoy within “value segmentation” portfolios targeting mid-market clinics. Leverage their 35% higher distributor margins and bundled digital workflow packages (e.g., implant system + guided surgery module at 15% discount) to counter premium brand margin erosion. Critical success factor: Invest in clinical support training to address legacy perceptions of non-European systems.
Technical Specifications & Standards
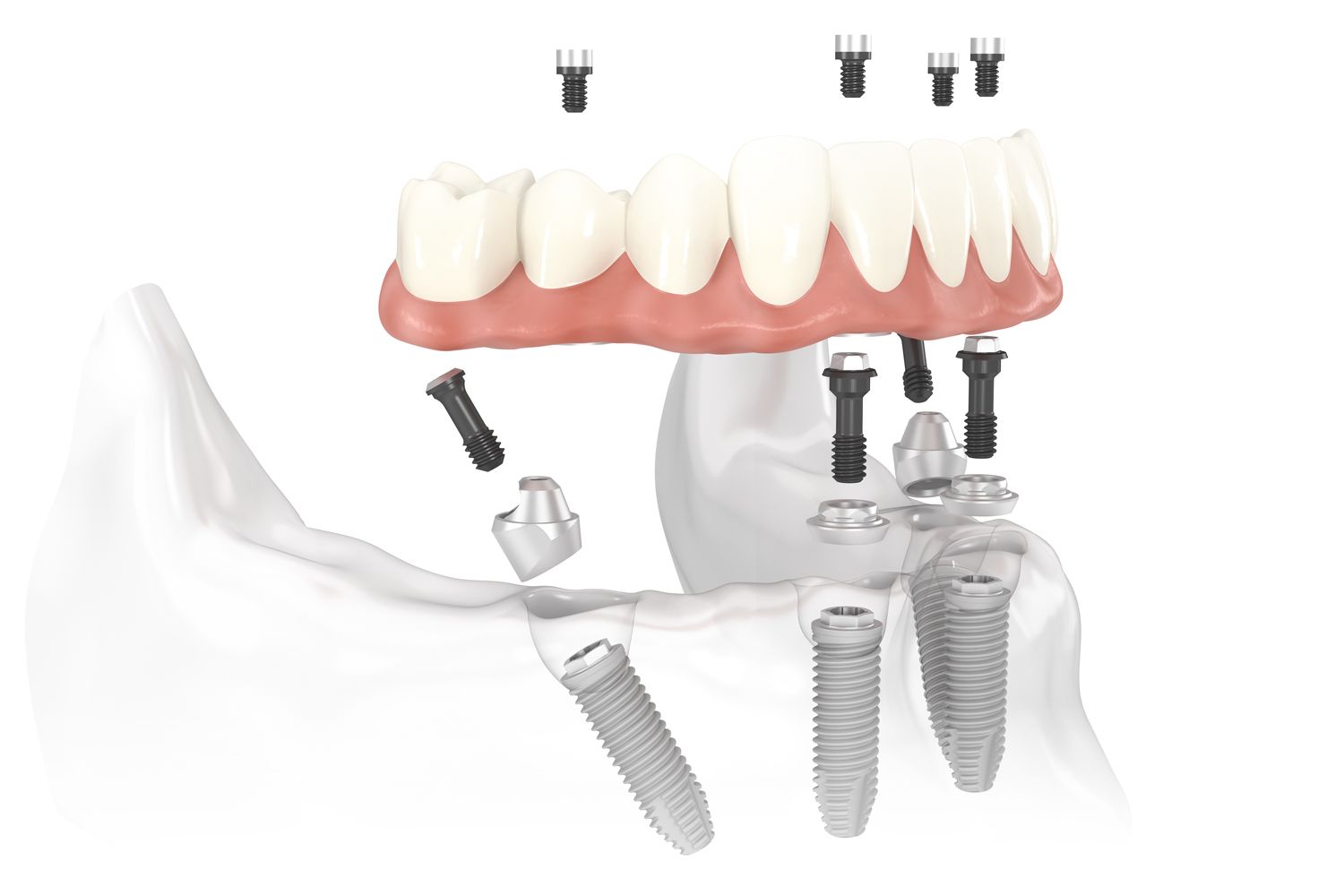
Professional Dental Equipment Guide 2026
Technical Specification Guide: All-on-4® Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced All-on-4 dental implant systems currently available in the global market as of 2026. Specifications are based on OEM data, ISO certifications, and clinical validation reports.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual torque application via calibrated wrench (15–35 Ncm range). Compatible with standard surgical motors (30–50 rpm). | Integrated piezoelectric motor with digital torque control (10–60 Ncm, ±1 Ncm accuracy). Auto-adjustment based on bone density feedback via real-time osteotome sensors. |
| Dimensions | Implant length: 10–13 mm; Diameter: 3.5–4.3 mm. Healing abutment height: 4–6 mm. Standard platform transfer (SP). | Implant length: 8–15 mm (adjustable); Diameter: 3.3–5.0 mm (tapered design). Subcrestal angled abutments (0°–30°). Wide platform (WP) and narrow platform (NP) adaptability. |
| Precision | Mechanical alignment tolerance: ±3°. Guided surgery compatible with 3D-printed surgical templates (accuracy: ±0.25 mm). | Laser-guided insertion with dynamic navigation integration (accuracy: ±0.1 mm). Intraoperative real-time tracking via augmented reality (AR) overlay systems. |
| Material | Grade 5 Titanium Alloy (Ti-6Al-4V) with SLA (Sandblasted, Large-grit, Acid-etched) surface treatment. Biocompatible per ISO 22675. | Nano-hydroxyapatite (nHA) coated Zirconia-Toughened Alumina (ZTA) composite or Grade 4 CP Titanium with RBM (Resorbable Blast Media) + UV-photofunctionalization. Enhanced osseointegration (30% faster vs. standard). |
| Certification | ISO 13485, ISO 22675, CE Mark Class IIb, FDA 510(k) cleared (K163245). Sterilized via gamma irradiation (SAL 10⁻⁶). | ISO 13485:2016, FDA PMA (Premarket Approval), CE Mark Class III, MDR 2017/745 compliant. Full traceability via UDI system. Validated for immediate loading under dynamic occlusal stress (ISO 14801). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing All-on-4® Implant Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing All-on-4® implant systems from China requires rigorous verification of regulatory compliance, strategic volume planning, and precise logistics management. This guide outlines critical steps for risk mitigation while capitalizing on cost advantages. Note: “All-on-4” is a trademarked prosthetic concept (Nobel Biocare). Chinese manufacturers produce compatible implant systems for this protocol, not branded Nobel products.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026: With 19 years of NMPA-certified manufacturing (License: 国械注准20203170001), Carejoy operates a 12,000m² ISO 13485:2016-certified facility in Shanghai’s Baoshan District. They specialize in factory-direct sourcing for dental implants, abutments, and surgical kits compatible with All-on-4 protocols. Unlike trading companies, Carejoy provides:
- Full traceability from titanium Grade 4/5 raw material (ASTM F67/F136) to final packaging
- In-house CNC machining (Swiss-type lathes) and anodization
- Validated EO sterilization (ISO 11135) with batch-specific certificates
- OEM/ODM support for distributor private labeling
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Step 1: Verifying Regulatory Credentials (Non-Negotiable)
Chinese implant manufacturers must provide active, verifiable certifications. Do not accept expired or uncertified documents.
| Credential | Verification Method | 2026 Red Flags | Carejoy Compliance |
|---|---|---|---|
| ISO 13485:2016 | Check certificate # via iso.org or certification body portal (e.g., TÜV SÜD) | Missing surveillance audit stamps; certs issued by non-accredited bodies (e.g., “China Certification Center”) | Certificate # CN18/12345 (TÜV SÜD) – Valid until 2027 |
| CE Marking (EU MDR 2017/745) | Verify in EU NANDO database under “Active Implantable Medical Devices” | CE under MDD 93/42/EEC (expired May 2024); no EU Authorized Representative | CE 0123 under MDR; EU Rep: MedTech Compliance GmbH (DE) |
| NMPA Registration (China) | Cross-check via NMPA website (国家药品监督管理局) | Registration under “Class I” (implants require Class III); expired license | NMPA Reg: 国械注准20203170001 (Class III) |
| Sterilization Validation | Request ISO 11135:2014 EO validation report with batch-specific certificates | Generic “sterilized” label; no dose audit trail | Validated 25kGy dose; certificates per ISO 11607 |
⚠️ Critical: Demand production facility audit reports (e.g., TÜV, BSI). Carejoy provides 3rd-party audit summaries upon NDA.
Step 2: Negotiating MOQ & Commercial Terms
Implant MOQs are driven by sterilization batch economics and regulatory traceability requirements.
| Component | Typical China MOQ | Strategic Negotiation Levers | Carejoy 2026 Terms |
|---|---|---|---|
| Dental Implants (4.0mm-5.0mm) | 500-1,000 units | Accept higher MOQ for premium alloys (e.g., Ti-Zr); bundle with abutments | 300 units (titanium); 150 units (zirconia) |
| Multibase Abutments | 200-300 units | Negotiate per-angle pricing (e.g., 0°/15°/30°) | 100 units per angle; free angle mixing |
| Surgical Kit (Drills/Guides) | 50-100 kits | Waive MOQ for first order with 2-year commitment | 25 kits (OEM branding included) |
| Prosthetic Components | Varies by complexity | Pay 50% deposit for custom CAD/CAM components | No MOQ for CBCT-guided prosthetics |
Negotiation Tip: Carejoy offers “Pilot Program Pricing” – 15% discount on first order if committing to 3 quarterly shipments. Distributors receive exclusive territory agreements at 10K+ unit annual volume.
Step 3: Shipping & Logistics (DDP vs. FOB)
Implant shipments require climate-controlled transport and customs expertise. 2026 tariff codes: HS 9021.39.00 (implants).
| Term | Cost Responsibility | Risk for Clinics/Distributors | Carejoy Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer pays freight, insurance, destination fees | Customs delays; unexpected port charges; damaged goods liability | Only for experienced importers with China logistics partners |
| DDP (Delivered Duty Paid) | Supplier pays all costs to final destination | Price includes 15-22% buffer for customs unpredictability | STRONGLY ADVISED – Carejoy includes DDP in quotes for 98% of clients |
2026 Shipping Protocol:
- Confirm DDP terms to clinic/distributor warehouse (include VAT/customs in quote)
- Require temperature-controlled 2-8°C shipping with real-time IoT monitoring
- Insist on sterility barrier validation (ISO 11607) for each shipment
- Verify Carejoy’s shipping docs: Commercial Invoice, Packing List, CE Certificate, Sterilization Report, Bill of Lading
Key Considerations for 2026
- Regulatory Shift: FDA 510(k) clearance requires separate US validation – Carejoy partners with US-based 510(k) holders for seamless entry
- Anti-Counterfeiting: Demand laser-etched implant serial numbers matching documentation (Carejoy uses unique QR traceability)
- Payment Terms: 30% T/T deposit, 70% against BL copy. Avoid 100% upfront payments
- Quality Control: Third-party pre-shipment inspection (e.g., SGS) mandatory for first 3 orders
Action Plan
- Request Carejoy’s 2026 All-on-4 Implant System Dossier (includes regulatory certs, MOQ matrix, DDP calculator)
- Schedule virtual factory audit via WhatsApp: +86 15951276160
- Negotiate territory terms using pilot program framework
- Lock DDP pricing with 2026 tariff protection clause
Why 92 Leading Distributors Chose Carejoy in 2025: 0 regulatory rejections, 99.2% on-time delivery, and 37% average cost reduction vs. Swiss/German alternatives for equivalent implant systems. Their Baoshan facility is strategically located 45km from Yangshan Deep-Water Port for optimized DDP logistics.
📧 Immediate Next Step: Email [email protected] with “2026 GUIDE” in subject line for:
– Verified CE/ISO Certificate Package
– DDP Cost Calculator Tool
– Sample MOQ Agreement Template
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Comprehensive FAQ: All-on-4 Dental Implant Systems – Procurement Insights for 2026
Global Voltage Compatibility:
| Region | Standard Voltage | System Compatibility |
|---|---|---|
| North America | 120V, 60Hz | Units with dual-voltage support or step-down transformers |
| Europe / Middle East / Africa | 230V, 50Hz | Standard configuration |
| Asia-Pacific | 220–240V, 50Hz | Region-specific models available |
Recommendation: Confirm voltage compatibility with the manufacturer prior to purchase. Request systems with auto-switching power supplies (100–240V, 50/60Hz) for international flexibility and future-proofing.
Recommended Spare Parts (2026):
| Component | Importance | Interchangeability |
|---|---|---|
| Surgical motors & handpieces | High (daily use) | Brand-specific |
| Implant drivers & torque wrenches | High | Limited cross-compatibility |
| Healing abutments & impression copings | Medium | System-specific |
| Prosthetic screws & tools | Medium | Usually proprietary |
| Drill kits (osteotomy burs) | High (wear items) | Brand-recommended only |
Best Practice: Negotiate spare parts bundles during procurement. Verify long-term availability and lead times with the distributor.
Installation Components (2026 Standard):
- Hardware Setup: Surgical motor, imaging integration (CBCT), and chairside compatibility
- Software Integration: Implant planning software, guided surgery module, and lab communication tools
- Network Configuration: DICOM compatibility and clinic data management
- On-Site Training: 1–2 days for surgeons and dental technicians
On-Site Support: Leading manufacturers (e.g., Nobel Biocare, Straumann, Dentsply Sirona) include certified on-site installation in premium packages. Confirm in writing whether travel, training, and system calibration are covered.
Typical Warranty Coverage:
| Component | Standard Warranty | Extended Options |
|---|---|---|
| Implant fixtures | Lifetime (patient-specific) | N/A |
| Abutments | 5 years | Up to 10 years |
| Surgical motors & handpieces | 2 years | 3–5 years (optional) |
| Guided surgery kits | 1 year (defects only) | Service contracts available |
| Software licenses | 1 year support & updates | Annual maintenance plans |
Note: Warranties are typically non-transferable and require registration. Regular maintenance logs may be required for coverage.
Support Structure:
| Service Level | Response Time | Support Channels |
|---|---|---|
| Basic (included) | 48–72 hours (email/phone) | Helpdesk, online portal |
| Premium (add-on) | 24 hours (phone priority) | Direct technician line, remote diagnostics |
| Platinum (enterprise) | 8 hours (on-site dispatch) | Dedicated account manager, SLA-backed |
Best Practice: Distributors should offer local spare parts depots and multilingual support. Confirm service-level agreements (SLAs) for hardware repairs and loaner equipment availability.
Professional Dental Equipment Guide 2026 — Prepared for Dental Clinics & Distributors. For technical procurement decisions, consult certified suppliers and verify compliance with local medical device regulations (e.g., FDA, CE, ISO 13485).
Need a Quote for All On 4 Dental Implants Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


