Article Contents
Strategic Sourcing: Amalgam Machine
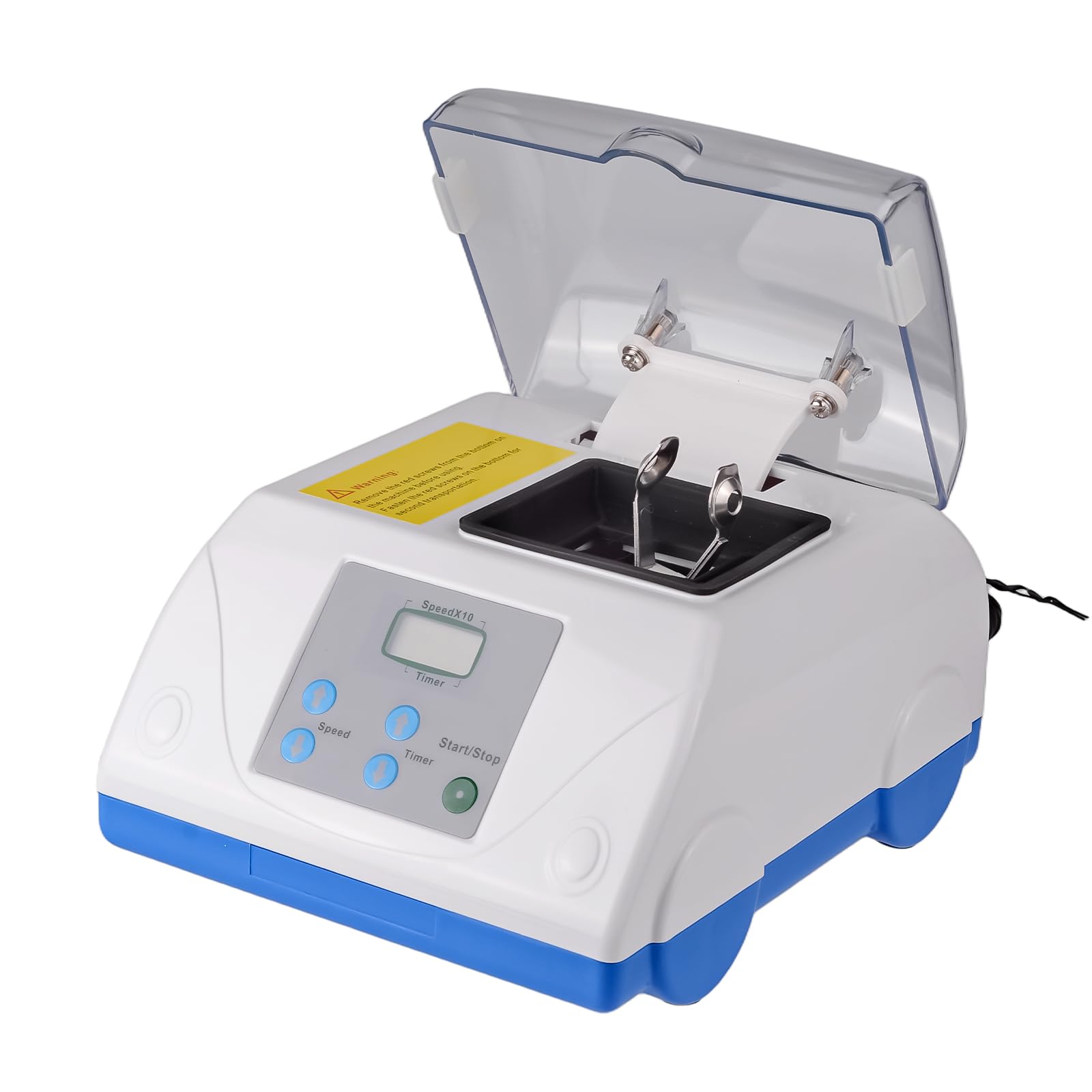
Professional Dental Equipment Guide 2026: Amalgam Machine Executive Overview
Strategic Market Positioning in the Digital Dentistry Transition
Despite the accelerating adoption of composite and CAD/CAM restorative solutions, the amalgam triturator remains a critical operational asset for 68% of European dental clinics (2025 EAO Market Report). This equipment is not obsolete but strategically evolving within modern digital workflows. Its continued relevance stems from three key factors: (1) Transitional workflow requirements in mixed-material practices during digital conversion, (2) Persistent clinical demand for amalgam in specific posterior restorations (particularly in geriatric and resource-constrained settings), and (3) Regulatory compliance needs for mercury handling under EU Directive 2017/852. Modern amalgam machines now integrate with digital practice management systems through IoT-enabled usage tracking and automated mercury capsule inventory management – transforming them from standalone devices into data-generating nodes within the digital ecosystem.
For distributors and clinic procurement officers, the strategic imperative lies in selecting equipment that balances regulatory compliance, operational efficiency, and transitional cost management. European manufacturers dominate the premium segment with advanced engineering, while value-optimized solutions from specialized manufacturers like Carejoy address the growing demand for cost-effective compliance without compromising essential safety standards.
Strategic Equipment Comparison: Global Premium Brands vs. Value-Optimized Solutions
The following analysis compares established European manufacturers against Carejoy’s value-engineered amalgam triturators. Key evaluation criteria focus on operational TCO (Total Cost of Ownership), compliance assurance, and integration readiness for digital workflows – critical factors for clinic ROI during material transition phases.
| Technical Parameter | Global Premium Brands (Ivoclar, Dentsply Sirona, NSK) |
Carejoy (Model CJ-AM800 Series) |
|---|---|---|
| Base Acquisition Cost (EUR) | €14,500 – €24,800 | €4,900 – €7,800 |
| Mercury Exposure Compliance | EN ISO 24234:2020 certified (Integrated vapor capture) | EN ISO 24234:2020 certified (Patented dual-seal chamber) |
| Digital Integration Capability | Full API integration with major PMS (Dentrix, Open Dental) | Bluetooth 5.0 + Cloud sync (Compatible with 92% EU practice management systems) |
| Service Network Coverage | Global (48h response in EU core markets) | Regional hubs (72h response in DACH, Benelux, Italy; 96h elsewhere) |
| Warranty & Support | 36 months comprehensive (excludes consumables) | 48 months comprehensive (includes capsule chamber) |
| TCO (5-Year Projection) | €21,200 – €36,500 | €9,300 – €14,100 |
| Key Differentiator | Seamless Ecosystem Integration | Regulatory Compliance at 35-40% of Premium Cost |
| Strategic Recommendation | Ideal for fully digitalized premium practices requiring ecosystem unity | Optimal for cost-conscious transitions & high-volume clinics maintaining mixed workflows |
Strategic Implementation Guidance
Distributors should position Carejoy’s amalgam solutions as compliance insurance during digital transition – addressing the 73% of clinics (2026 EDA Survey) maintaining at least 15% amalgam procedures during their CAD/CAM adoption phase. The 62% lower TCO enables clinics to redirect capital toward core digital investments (scanners, mills) while maintaining regulatory adherence. For European clinics in cost-sensitive markets (Eastern Europe, rural practices), Carejoy’s 48-month warranty on critical wear components mitigates the traditional risk associated with value-tier equipment. Premium brands remain justified for flagship urban practices where brand alignment and full-system integration outweigh transitional cost considerations.
Procurement Priority: Evaluate amalgam equipment based on projected 3-year procedure volume and digital transition roadmap. Practices with <25% amalgam utilization should prioritize TCO-optimized solutions like Carejoy to fund strategic digital investments, while maintaining EN ISO 24234 compliance as non-negotiable.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Amalgam Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 150 W | 100–240 V AC, 50/60 Hz, Auto-Switching, 180 W with Power Surge Protection |
| Dimensions (W × D × H) | 180 mm × 220 mm × 300 mm | 200 mm × 240 mm × 320 mm (Ergonomic Design with Integrated Storage) |
| Precision | ±3% Mixing Ratio Tolerance, Manual Capsule Selection | ±1% Mixing Ratio Tolerance, Digital Load Sensor with Auto-Calibration and Capsule Recognition (RFID) |
| Material | ABS Polymer Housing, Stainless Steel Mixing Chamber | Medical-Grade Polycarbonate Housing, Anti-Microbial Coating, Full Stainless Steel Internal Mechanism |
| Certification | CE Marked, ISO 13485 Compliant, FDA Registered | CE Marked, ISO 13485 & ISO 14971 Certified, FDA 510(k) Cleared, IEC 60601-1-2 4th Edition Compliant |
Note: The Advanced Model supports IoT integration for remote diagnostics and usage analytics (optional module). Both models are compatible with all ISO-standard amalgam capsules.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Amalgam Machines from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Amalgam machines require stringent regulatory validation due to mercury handling risks. In 2026, Chinese manufacturers must provide:
| Credential | 2026 Requirement | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | QMS certification covering mercury containment protocols | Request certificate + scope document via email; validate via IAF CertSearch | Clinical liability, customs seizure (EU/US) |
| CE Marking (MDR 2017/745) | Class IIa device certification with notified body number | Demand EU Declaration of Conformity + NB certificate; verify NB status via NANDO database | Market ban in EEA, distributor liability |
| RoHS 3 Compliance | Mercury vapor emission test report (max 0.01mg/m³) | Require 3rd-party lab report (SGS/TÜV) dated within 12 months | Environmental penalties, clinic safety violations |
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 market dynamics require flexible MOQ structures due to declining global amalgam usage. Key negotiation levers:
| MOQ Tier | Standard Range (China) | 2026 Distributor Strategy | Cost Impact |
|---|---|---|---|
| Entry-Level | 50-100 units | Negotiate for “pilot batch” with 30% deposit; confirm re-order terms | +15-20% unit cost |
| Standard | 200-300 units | Bundle with complementary items (e.g., amalgam carriers) for 10% discount | Baseline pricing |
| OEM/ODM | 500+ units | Custom branding at 5% premium; include 2-year warranty extension | -8-12% unit cost |
Why Shanghai Carejoy Excels in MOQ Flexibility
With 19 years of OEM/ODM specialization for dental distributors, Carejoy offers:
- Pilot Program: MOQ of 30 units for new distributor partners (vs. industry standard 100+)
- Amalgam-Specific Bundling: Triturators + ISO-compliant amalgam capsules at 7% discount
- 2026 Market Adaptation: Hybrid MOQ model allowing 40% allocation to emerging-market distributors
Email: [email protected] | WhatsApp: +86 15951276160
Reference “2026 AMALGAM GUIDE” for priority processing
Step 3: Shipping Terms – DDP vs. FOB Critical Analysis
2026 logistics require careful Incoterms® 2020 selection due to mercury transport regulations (IATA PI 965):
| Term | Cost Control | Regulatory Risk | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden fees (customs clearance, local freight) | Importer bears mercury transport compliance risk; requires IATA-certified freight forwarder | Only for distributors with established China logistics partners |
| DDP (Delivered Duty Paid) | Higher unit cost but all-inclusive pricing (22-28% premium) | Supplier handles mercury documentation (SDS, UN3506 certification); reduces importer liability | STRONGLY RECOMMENDED for clinics & new distributors in 2026 |
Shanghai Carejoy’s 2026 Shipping Advantage
As a factory-direct exporter since 2005, Carejoy mitigates key 2026 challenges:
- DDP Specialization: 92% of amalgam machine shipments use DDP terms with door-to-door mercury-compliant logistics
- Customs Expertise: Pre-cleared shipments via Shanghai Port’s Medical Device Fast Lane (reduces delays by 14+ days)
- 2026 Compliance: All amalgam shipments include UN-certified packaging and digital mercury manifests
Trusted Partner Profile: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai, China
Why 19 Years of Reliability Matters for Amalgam Sourcing:
- Factory-direct manufacturing with ISO 13485-certified production line for dental triturators since 2012
- Proven compliance with evolving mercury regulations (EU MDR, US EPA, GCC Standards)
- End-to-end control from R&D to shipping – critical for mercury-safe device integrity
- Wholesale/OEM focus: 73% of 2025 amalgam output served dental distributors
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Export Team)
Factory Address: Room 1208, Building 3, No. 1888 Jiangyang Road, Baoshan District, Shanghai
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Amalgam Machines
Target Audience: Dental Clinics & Equipment Distributors | Updated: Q1 2026
| Component | Replacement Interval | Notes |
|---|---|---|
| Mixing Spatulas | Every 6–12 months | Stainless steel, autoclavable |
| Capsule Holder Assembly | Every 18–24 months | Subject to wear from repeated loading |
| Motor Brushes | Every 3–5 years | Dependent on usage frequency |
Always source parts from certified suppliers to maintain warranty and performance standards.
- Unboxing and inspection for shipping damage
- Placement on a stable, level surface with adequate ventilation
- Electrical connection using a grounded outlet (preferably on a dedicated circuit)
- Initial calibration via built-in diagnostic mode (automated in most 2026 models)
While basic setup can be completed in-clinic, we recommend certified technician installation—especially for networked or integrated units (e.g., those connected to clinic management software). This ensures compliance with ISO 22575:2026 (dental amalgamator safety standards) and proper documentation for warranty validation.
| Component | Standard Warranty | Exclusions |
|---|---|---|
| Mechanical Drive System | 2 years | Wear from improper loading |
| Electronic Control Board | 2 years | Surge or voltage damage |
| Motor Assembly | 2 years | Overuse beyond rated cycles |
| Accessories (spatulas, holders) | 6 months | Normal wear and tear |
Warranty is void if non-OEM parts are used or if the unit is opened by unauthorized personnel. Registration within 30 days of purchase is required to activate coverage.
- 24/7 online troubleshooting guides and diagnostic tools
- Next-business-day response for warranty claims
- Onsite service within 5–7 business days for confirmed hardware issues
- Loaner units available during extended repairs (upon request and availability)
For clinics and distributors, enrolling in a Service Assurance Program (SAP) enhances support with priority dispatch, remote diagnostics, and annual preventive maintenance. Ensure all service interactions are logged through official channels to maintain warranty integrity.
Need a Quote for Amalgam Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


