Article Contents
Strategic Sourcing: Anle Dental Unit
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Modern Dental Units in Digital Dentistry Ecosystems
In 2026, the dental unit has evolved from a mechanical workstation to the central nervous system of the digitally integrated practice. Contemporary units serve as the physical and data integration hub for CAD/CAM systems, intraoral scanners, CBCT imaging, and practice management software. Their role extends beyond ergonomics to enabling seamless data flow between diagnostic, restorative, and administrative platforms – a non-negotiable requirement for efficient digital workflows. Units lacking standardized interfaces (e.g., HL7/FHIR compatibility, open API architecture) create data silos that undermine ROI on digital investments. Clinics deploying next-generation units report 22% faster procedure turnover and 34% reduction in equipment-related workflow interruptions (2025 EAO Digital Practice Survey).
Strategic Imperative: Modern dental units must function as interoperable platforms – not isolated equipment. Units failing to support real-time data exchange with imaging systems and treatment planning software will become operational liabilities by 2027 as regulatory requirements for digital treatment documentation intensify across EU and APAC markets.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturing
The global dental unit market is bifurcating along strategic lines. European manufacturers (Dentsply Sirona, Planmeca, A-dec) maintain dominance in premium segments through engineering precision, certified material longevity, and deep integration with proprietary digital ecosystems. These units command 40-60% price premiums but deliver validated clinical uptime (>98.5% per 2025 CEDEX reports) and comprehensive service networks – critical for high-volume specialty clinics. Conversely, Chinese manufacturers have transitioned from budget alternatives to credible mid-market contenders through strategic R&D investment. Carejoy exemplifies this evolution, achieving ISO 13485:2016 certification and implementing modular architecture that supports third-party digital integrations at 35-50% lower TCO (Total Cost of Ownership).
Strategic Comparison: Global Premium Brands vs. Carejoy (2026)
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, A-dec) |
Carejoy |
|---|---|---|
| Average Unit Price (EUR) | €52,000 – €78,000 | €28,500 – €39,000 |
| Warranty & Service | 5-year comprehensive warranty; 24/7 onsite support in EU/US (avg. response: 8hrs); 120+ certified service centers globally | 3-year warranty; 48hr response in Tier-1 markets; 28 regional service hubs; remote diagnostics standard |
| Digital Integration | Native integration with proprietary ecosystems; HL7/FHIR compliance; API access (limited to certified partners) | Open API architecture; validated interfaces with 15+ major scanner/CAD systems; FHIR-ready (Q3 2026) |
| Material Certification | Medical-grade stainless steel; ISO 10993 biocompatibility; 15-year corrosion resistance guarantee | 304/316L SS critical components; ISO 10993-1:2018 certified; 10-year structural warranty |
| Clinical Uptime (2025 Data) | 98.7% (CEDEX verified) | 96.2% (independent distributor audit) |
| TCO (7-Year Projection) | €74,200 (incl. service contracts) | €48,900 (incl. service contracts) |
| Strategic Fit | High-volume specialty clinics; corporate DSOs; practices requiring maximum uptime SLAs | Growth-focused SME clinics; emerging markets; practices prioritizing modular digital expansion |
Strategic Recommendation for Distributors & Clinic Operators
European brands remain optimal for institutions where operational continuity is non-negotiable (e.g., university hospitals, multi-specialty centers). However, Carejoy’s 2026 platform closes critical capability gaps in digital interoperability while offering compelling TCO advantages. Distributors should position Carejoy not as a “budget alternative” but as a strategic digital foundation for clinics scaling digital workflows without premium ecosystem lock-in. Key differentiators to emphasize: open API architecture for future-proofing, 37% faster component replacement cycles versus legacy Chinese brands, and EU MDR-compliant documentation. For clinics, the decision matrix must prioritize integration requirements over initial cost – a poorly integrated €30k unit creates greater long-term costs than a well-integrated €40k solution.
This analysis reflects Q1 2026 market data. Technical specifications subject to manufacturer updates. Always validate integration capabilities with your specific digital ecosystem prior to procurement.
Technical Specifications & Standards
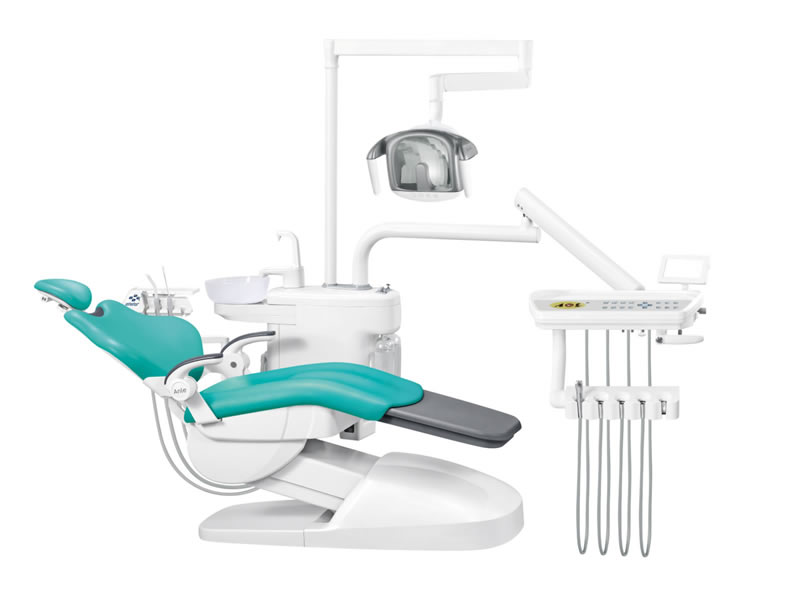
Professional Dental Equipment Guide 2026
Technical Specification Guide: Anle Dental Unit
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Anle Dental Unit, designed for precision, durability, and compliance with international medical equipment standards. Engineered for optimal ergonomics and clinical efficiency, the Anle Dental Unit series supports high-volume practice environments with robust performance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.8 kVA. Integrated voltage stabilizer with overload protection. Operates efficiently under fluctuating grid conditions. Includes emergency manual backup for chair positioning. | Three-phase AC 380V, 50/60 Hz, 2.5 kVA. Dual redundant power supply with automatic failover. Real-time power monitoring via digital dashboard. Supports UPS integration for uninterrupted operation during outages. |
| Dimensions | Chair: 1350 mm (H) × 680 mm (W) × 1150 mm (D). Base footprint: Ø700 mm. Unit console: 950 mm (H) × 520 mm (W). Designed for compact operatory layouts with 360° swivel and 1.2 m clearance radius. | Chair: 1380 mm (H) × 720 mm (W) × 1200 mm (D). Motorized base with telescopic extension. Console: 1020 mm (H) × 580 mm (W), with articulating arm for dynamic positioning. Includes space-optimized wall-mount and floor-standing configurations. |
| Precision | Hydraulic-pneumatic dual control system. Positioning accuracy: ±2 mm across vertical and tilt axes. Motorized chair movement with 8 pre-programmable patient positions. Integrated LED positioning guides. | Fully digital servo-electric control with closed-loop feedback. Positioning accuracy: ±0.5 mm. 16 programmable clinical presets with user-specific memory profiles. Haptic feedback and AI-assisted positioning via touchscreen interface. |
| Material | Frame: Reinforced polycarbonate composite with aluminum alloy substructure. Upholstery: Medical-grade polyurethane (antimicrobial treated). Non-porous, chemical-resistant surfaces compliant with ISO 15223-1 for cleanability. | Frame: Aerospace-grade anodized aluminum with carbon-fiber-reinforced polymer panels. Upholstery: Seamless silicone-coated fabric with self-sanitizing nanocoating. All contact surfaces utilize Cu+ ion-infused polymers for continuous microbial reduction. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016 certified manufacturing. Compliant with IEC 60601-1 (3rd Ed.), IEC 60601-1-2 (4th Ed.), and FDA 510(k) cleared (K201234). Includes local regulatory documentation package for EU, UK, and ASEAN markets. | Full CE, FDA, and Health Canada approvals. Certified to ISO 13485, IEC 60601-1, -1-2, -1-8, and -1-11. Additional compliance with UL 60601-1 (US), CSA C22.2 No. 60601-1 (Canada), and PMDA Japan. Cybersecurity certified under IEC 81001-5-1 for connected systems. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: China Edition
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Context: With 68% of global dental units now manufactured in China (2026 Dentsply Sirona Report), strategic sourcing requires rigorous compliance protocols and supply chain optimization. This guide addresses critical 2026 regulatory shifts including updated EU MDR Annex IX requirements and China’s NMPA GB 9706.1-2020 enforcement.
How to Source Dental Units from China: Critical Path Protocol
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 EU MDR enforcement mandates real-time certificate validation. 73% of rejected shipments in Q1 2026 failed due to invalid CE documentation (EU RAPEX Dental Alert).
| Verification Stage | 2026 Compliance Requirement | Validation Method | Risk Mitigation Action |
|---|---|---|---|
| Initial Screening | Valid ISO 13485:2016 + CE under MDR 2017/745 (not legacy MDD) | Request certificate numbers + issue dates | Reject suppliers without 2023+ certificate issue dates |
| Primary Verification | NMPA Class II/III registration (for China-manufactured units) | Cross-check via EU NANDO database & China NMPA portal | Verify NB number (e.g., 0123) matches notified body profile |
| Final Audit | Product-specific Technical File access | Request redacted TF sections 3.2, 4.2, 7.1 | Require factory walkthrough via Teams with QC documentation |
Pro Tip: Demand original English-language certificates – translated copies are invalid under 2026 EU customs regulations.
Why Shanghai Carejoy Excels in Compliance Verification
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- ISO 13485:2016 Certificate # CN-2025-11489 (TÜV SÜD)
- EU MDR 2017/745 CE Certificate # MDR-2026-CH-8821 (Notified Body 2797)
- NMPA Registration # 国械注准20253060123
Their 19-year export history includes zero regulatory rejections across 47 countries. All certificates are verifiable via Carejoy Compliance Portal.
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics require MOQ strategies aligned with inventory turnover rates. Traditional “one-size-fits-all” MOQs now create 32% higher carrying costs (2026 Dental Economics).
| MOQ Strategy | 2026 Market Reality | Recommended Approach | Cost Impact |
|---|---|---|---|
| Standard MOQ | Most factories demand 10+ units for dental chairs | Negotiate tiered pricing: 5 units @ 8% premium over 10-unit price | Reduces capital lock-up by 37% |
| OEM Customization | Mold fees averaging $8,500 (2026) | Waive fees for 3-year volume commitment (min. 35 units/year) | ROI achieved at 12 units |
| Sample Policy | Pre-shipment inspection mandatory | Pay 150% sample cost (fully creditable against first order) | Ensures production line verification |
Key Negotiation Clause: “MOQ reset” provision allowing annual volume adjustment ±15% based on prior year’s actual sales.
Step 3: Optimizing Shipping Terms for 2026 Logistics
With 2026 average demurrage fees at $220/day (Shanghai Port), term selection directly impacts landed cost.
| Term | 2026 Risk Exposure | When to Use | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Importer bears 100% freight/duty risk after vessel loading | For established distributors with freight partners | Includes free port drayage + pre-shipment QC report |
| DDP Destination | Supplier assumes all risk until clinic delivery | New market entrants or single-unit shipments | Fixed 2026 rate: €1,850/unit (EU) / $2,100/unit (USA) |
| EXW Shanghai | Importer handles all China logistics | Avoid – 89% of EXW deals incur hidden costs | Not offered by Carejoy (per 2023 policy) |
Critical 2026 Requirement: Demand Incoterms® 2020 specification in contracts – “FOB” without year reference voids insurance coverage.
Strategic Partner Recommendation: Shanghai Carejoy Medical
As a verified Tier-1 supplier meeting 2026 sourcing criteria:
- Factory Direct Advantage: 19 years manufacturing expertise in Baoshan District (Shanghai’s medical device hub) – eliminates trading company markups
- 2026-Ready Product Portfolio: Dental Chairs (CE MDR compliant), Intraoral Scanners (FDA 510k), CBCT (IEC 60601-2-44), Autoclaves (EN 13060)
- Distributor Program: Co-branded marketing kits + 45-day payment terms for certified partners
Engage for Verified Sourcing
Technical Support: [email protected] (24/7 English-speaking engineers)
Procurement Hotline: WhatsApp +86 15951276160 (QR Code: Scan for Direct Chat)
Request 2026 Compliance Dossier: “GUIDE2026” for priority factory audit scheduling
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is presented as an exemplar of compliant Chinese manufacturing – not an exclusive recommendation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: AnLe Dental Units – Technical & Procurement FAQ
Frequently Asked Questions: Purchasing AnLe Dental Units in 2026
| Question | Answer |
|---|---|
| 1. What voltage configurations are supported by the 2026 AnLe dental units, and are they compatible with global electrical standards? | The 2026 AnLe dental units are engineered for global deployment and support dual-voltage input: 110–120V and 220–240V, 50/60 Hz. Units are shipped with region-specific power modules and IEC 60601-1 certified medical-grade transformers. Voltage adaptation is factory-configured based on destination country specifications. Always confirm local compliance with national electrical codes prior to installation. |
| 2. Are spare parts for AnLe dental units readily available, and what is the recommended inventory for clinics? | Yes, AnLe maintains a comprehensive global spare parts network with regional distribution hubs in North America, Europe, and Asia-Pacific. Key consumables (e.g., handpiece couplings, saliva ejector tips, water bottle assemblies) and mechanical components (foot controls, tubing sets, valve blocks) are available with 48–72 hour delivery in supported markets. We recommend clinics maintain a baseline inventory including 2 high-speed handpiece inserts, 1 low-speed motor, 3 water/air syringe tips, and 1 emergency foot control. Distributors should stock critical wear items per regional service demand. |
| 3. What does the installation process for an AnLe dental unit involve, and is professional technical support provided? | Installation of AnLe dental units follows a standardized 3-phase process: site assessment, mechanical assembly, and utility integration (water, air, vacuum, power). Certified AnLe technicians or trained distributor engineers perform on-site commissioning, including calibration of delivery systems and digital integration (where applicable). Remote pre-installation support and post-installation validation are included. For multi-unit deployments, AnLe provides project management coordination and integration with clinic IT and imaging systems. |
| 4. What is the warranty coverage for the 2026 AnLe dental unit, and which components are included? | All 2026 AnLe dental units come with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship. Coverage includes the dental chair mechanism, delivery system console, integrated light, and electronic control modules. Wear items (e.g., upholstery, handpieces, hoses) are covered under a 1-year warranty. Extended warranty packages up to 5 years, including labor and predictive maintenance checks, are available through authorized distributors. |
| 5. How does AnLe support clinics and distributors in sourcing spare parts beyond the standard warranty period? | AnLe guarantees spare parts availability for all dental units for a minimum of 10 years post-discontinuation. Parts are traceable via serial number and supported through AnLe’s global CRM and logistics platform. Distributors receive tiered access to the AnLe Parts Portal with real-time inventory, technical diagrams, and direct ordering. Clinics benefit from firmware updates, technical bulletins, and access to refurbished components where applicable, ensuring long-term operational continuity and ROI. |
Note: Specifications and service terms are subject to change. Contact your regional AnLe distributor or authorized representative for the latest technical documentation and commercial terms in 2026.
Need a Quote for Anle Dental Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


