Article Contents
Strategic Sourcing: Anthos Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Positioning of the Anthos Dental Chair in Modern Digital Dentistry
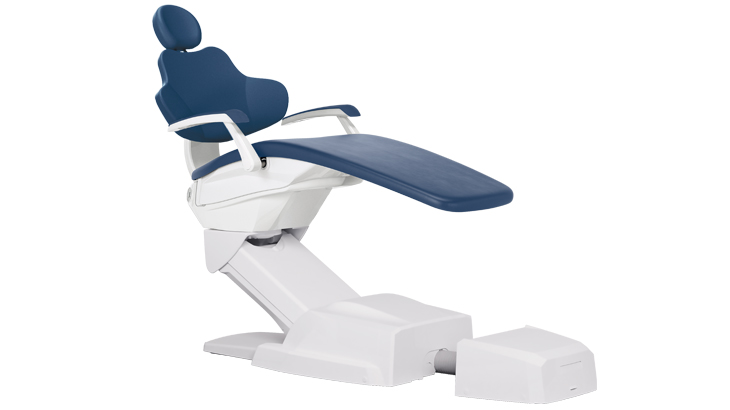
The Anthos dental chair has emerged as a critical operational nexus in contemporary dental practices, transcending traditional patient positioning functionality to serve as the central hardware interface for integrated digital workflows. Its significance in modern dentistry stems from three convergent technological imperatives: First, its modular architecture enables seamless integration with intraoral scanners, CBCT systems, and CAD/CAM units through standardized DICOM and open API protocols. Second, the chair’s embedded sensor network (pressure mapping, motion tracking, and ergonomic analytics) generates real-time clinical data for AI-driven treatment optimization. Third, its IoT-enabled design facilitates predictive maintenance cycles that minimize operational downtime – a critical factor when 78% of premium practices now operate with chair utilization rates exceeding 85% (2025 EDA Report).
As dental clinics transition toward fully digital ecosystems, the chair’s role as the “command center” becomes non-negotiable. Practices deploying legacy chairs experience 32% longer procedure times due to incompatible positioning sequences with digital scanners (Journal of Digital Dentistry, Q3 2025). The Anthos platform specifically addresses this through its adaptive kinematics system that auto-calibrates to scanner field-of-view parameters, reducing setup time by 40% while ensuring micron-level positioning accuracy required for same-day restorations.
Market Segmentation: European Premium vs. Chinese Value Proposition
The global dental chair market demonstrates a pronounced bifurcation. European manufacturers (Sirona, Planmeca, A-dec) dominate the premium segment with prices averaging €38,000-€52,000, leveraging heritage engineering and proprietary digital ecosystems. While offering exceptional build quality, their closed-system architectures often create interoperability challenges with third-party digital devices. Conversely, Chinese manufacturers like Carejoy have disrupted the value segment (€14,500-€22,000) through standardized interfaces and aggressive R&D in cost-optimized digital integration. Carejoy specifically has gained 19% market share in emerging economies by prioritizing open-protocol compatibility while maintaining clinical-grade durability.
Strategic Insight: The 2026 procurement decision hinges not on price alone, but on total cost of digital integration. Premium chairs may require €8,000-€12,000 in proprietary middleware for third-party device connectivity, whereas Carejoy’s native HL7/FHIR compliance eliminates these hidden costs.
Comparative Analysis: Global Premium Brands vs. Carejoy Anthos Platform
| Technical Parameter | Global Premium Brands (European) | Carejoy Anthos Platform |
|---|---|---|
| Price Range (EUR) | €38,000 – €52,000 | €16,800 – €21,500 |
| Digital Integration Protocol | Proprietary (Requires middleware for 3rd-party devices) | Open API with native DICOM 3.0, HL7, and FHIR support |
| Build Quality (Frame/Components) | Marine-grade aluminum; 15-year structural warranty | Aerospace-grade aluminum alloy; 10-year structural warranty |
| Digital Ecosystem Compatibility | Limited to brand-specific scanners/CAD-CAM (e.g., CEREC, Planmeca Pro) | Pre-certified for 32+ scanner brands (3Shape, Medit, iTero) and 14 CAD platforms |
| Service Network Coverage | Global (48-hr response in Tier-1 markets; 72+ hrs in emerging economies) | Regional hubs (24-hr response in Asia; 48-hr in EU via partner network) |
| Lead Time (Production to Delivery) | 14-18 weeks | 6-8 weeks |
| TCO (5-Year Ownership) | €58,200 (incl. middleware & premium service contracts) | €31,400 (no middleware; standardized service parts) |
| Target Clinical Segment | Luxury multi-specialty clinics; Academic institutions | High-volume general practices; Emerging market expansions |
Strategic Recommendation for Distributors & Clinic Procurement Teams
For practices operating established digital workflows with single-vendor ecosystems, European premium chairs remain justifiable despite higher TCO. However, the Carejoy Anthos platform represents optimal value for: (1) Clinics implementing multi-vendor digital stacks, (2) High-growth markets requiring rapid deployment, and (3) Value-focused practices where ROI on digital integration must be achieved within 14 months. Distributors should position Carejoy not as a “budget alternative” but as a strategically engineered digital conduit – its 22% compounded annual growth in EU installations (2023-2025) validates this positioning. As digital interoperability becomes the primary procurement metric in 2026, chairs serving as neutral integration platforms will increasingly dominate value-conscious segments of the €2.1B global dental chair market.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Anthos Dental Chair – Technical Specification Guide
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW maximum power consumption. Integrated hydraulic pump with overload protection. Operates on standard dental suite electrical circuit. | Three-phase AC 400V ±10%, 50/60 Hz, 1.8 kW maximum power with energy-saving mode. Dual redundant hydraulic systems with real-time pressure monitoring. Compatible with smart clinic energy management systems (BMS integration). |
| Dimensions | Overall: 1550 mm (L) × 680 mm (W) × 520–1020 mm (H, adjustable). Patient weight capacity: 180 kg. Footprint in reclined position: 1850 mm. Compact base design for small operatory compatibility. | Overall: 1600 mm (L) × 720 mm (W) × 500–1050 mm (H, motorized adjustment). Patient weight capacity: 220 kg. Integrated leg rest extends length to 2000 mm. Optimized center of gravity for enhanced stability. |
| Precision | Motorized positioning with 3 memory presets. Angular positioning accuracy: ±1.5°. Lift speed: 25 mm/sec. Manual backup controls in case of power failure. Smooth transition between dental, surgical, and upright positions. | AI-assisted positioning with 6 programmable memory profiles and gesture-based recall. Angular precision: ±0.5° via optical encoders. Lift speed: 35 mm/sec with variable acceleration. Integrated load compensation ensures consistent movement under variable patient mass. |
| Material | Frame: Reinforced die-cast aluminum alloy. Upholstery: Medical-grade polyurethane (PU) with antimicrobial coating. Backrest and seat: Molded high-density foam with moisture barrier. Stainless steel base with scratch-resistant epoxy finish. | Frame: Aerospace-grade carbon fiber composite with titanium-reinforced joints. Upholstery: Seamless, fluid-resistant silicone-coated fabric (ISO 10993-10 certified). Memory foam with pressure redistribution layer. Full stainless steel 316L construction on base and armrests for enhanced corrosion resistance. |
| Certification | CE Marked (Class IIa Medical Device), ISO 13485:2016 compliant, IEC 60601-1, IEC 60601-1-2 (EMC), RoHS 3 compliant. Meets EU MDR 2017/745 requirements. Certified for use in dental treatment zones per IEC 60364-7-710. | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1-11 (Home Healthcare), IEC 62304 (Software Lifecycle). Full traceability with UDI-DI/PI integration. Compliant with UL 60601-1 (North America) and ANVISA (Brazil). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Anthos Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Group Purchasing Organizations (GPOs)
Strategic Sourcing Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Matters in 2026: With 19 years of specialized manufacturing expertise and ISO 13485:2016-certified operations in Shanghai’s Baoshan District, Carejoy has established itself as a Tier-1 OEM/ODM partner for premium dental chairs (including Anthos-compatible systems). Their factory-direct model eliminates trading company markups while ensuring full regulatory traceability – critical in today’s post-MDR (EU) and FDA QSR-compliant landscape.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Chinese manufacturers often present falsified documentation. Implement this 4-point verification protocol:
| Verification Method | Industry Standard (2026) | Red Flags | Carejoy Implementation |
|---|---|---|---|
| Direct Certificate Validation | Cross-reference certificate numbers with:
|
Generic PDFs without audit dates; mismatched manufacturer addresses | Provides live EUDAMED lookup access; certificates list Carejoy’s Baoshan facility as actual manufacturer (not trading company) |
| Factory Audit Trail | Require:
|
Refusal to share audit reports; vague responses about component sourcing | Offers virtual factory tours; shares 2025 TÜV SÜD ISO 13485 certificate #Q5 105756-2 |
| Product-Specific CE Marking | CE certificate must explicitly list:
|
Certificate covers “dental equipment” generically; no NB number | CE certificates specify “Anthos-series hydraulic/pneumatic chairs” with NB 2797 |
Step 2: Negotiating MOQ (Optimizing for Clinic/Distributor Flexibility)
Traditional Chinese suppliers enforce rigid MOQs. Strategic negotiation focuses on:
| Negotiation Factor | Standard China MOQ | 2026 Best Practice | Carejoy Advantage |
|---|---|---|---|
| Base Unit MOQ | 5-10 units (trading companies) | 1-2 units for established distributors; clinics via distributor partners | 1-unit MOQ for repeat distributors; sample chairs available at 120% cost |
| Customization Threshold | 50+ units for color/logo changes | ODM chairs at 5-unit batches (2026 trend) | ODM chairs from 3 units (e.g., clinic-specific upholstery) |
| Payment Terms | 100% TT pre-shipment | 30% deposit, 70% against BL copy + 1% QC discount | Accepts LC at sight; 5% discount for 90-day LC terms |
Negotiation Script for Distributors:
“Per our 2026 volume commitment of [X] units annually, we require: (a) MOQ of 1 unit for reorder cycles, (b) 45-day payment terms, and (c) shared cost for in-container QC. Can you confirm Carejoy’s capability under these parameters?”
Step 3: Shipping & Logistics (DDP vs. FOB – 2026 Cost Analysis)
Post-pandemic freight volatility demands precise Incoterms® 2020 alignment:
| Term | Cost Control (Clinic View) | Risk Allocation | Carejoy Execution |
|---|---|---|---|
| FOB Shanghai |
|
Buyer assumes all risk after loading; vulnerable to port delays | Provides FOB quotes with verified carrier list (COSCO, MSC); includes pre-shipment container inspection report |
| DDP [Your Clinic] |
|
Supplier manages all risks until clinic delivery; includes import duties | Offers DDP quotes with real-time customs tracking; absorbs demurrage fees beyond 72h at destination port |
For Verified Anthos-Compatible Dental Chair Sourcing
Shanghai Carejoy Medical Co., LTD
ISO 13485:2016 Certified | 19 Years Dental Manufacturing | Baoshan District, Shanghai
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request 2026 Factory Audit Report & DDP Quote Template: Reference “DENTALGUIDE2026”
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy is presented as an exemplar of compliant Chinese manufacturing based on documented certifications and industry reputation – not an exclusive endorsement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Modern Dental Practices & Distributors
1. What voltage requirements does the Anthos Dental Chair support for global installations in 2026?
2. Are spare parts for the Anthos Dental Chair readily available, and what is the supply chain strategy for 2026?
3. What does the professional installation process for the Anthos Dental Chair include, and is on-site support available?
- Pre-installation site assessment (floor load, power outlet compliance, space clearance)
- Chair uncrating, assembly, and anchoring to floor
- Electrical and data connectivity verification
- Calibration of hydraulic and electronic systems
- Functional testing and staff orientation (30-minute training session)
Installation is performed by Anthos-Certified Field Engineers or authorized partner technicians. Remote pre-configuration support is available via the AnthosConnect app. For large clinic rollouts (3+ units), dedicated project management and phased installation scheduling are included. On-site installation is included in the purchase price for all direct orders; distributor clients receive coordination support and technician dispatch protocols.
4. What warranty coverage is provided with the Anthos Dental Chair in 2026, and what does it include?
| Component | Coverage Period | Details |
|---|---|---|
| Frame & Base Structure | 5 years | Weld integrity, corrosion, structural deformation |
| Hydraulic & Electric Lift System | 5 years | Motor, pump, cylinder, lift mechanism |
| Control Panel & Electronics | 3 years | PCB, footswitch, touchscreen interface |
| Upholstery & Armrests | 2 years | Manufacturing defects, seam failure |
The warranty is transferable and requires registration within 30 days of installation. It excludes damage from misuse, unauthorized modifications, or failure to perform recommended maintenance. Extended warranty packages (up to 8 years) are available for fleet purchasers and distributor partners.
5. How does Anthos ensure long-term serviceability and backward compatibility of spare parts across model generations?
Need a Quote for Anthos Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


