Article Contents
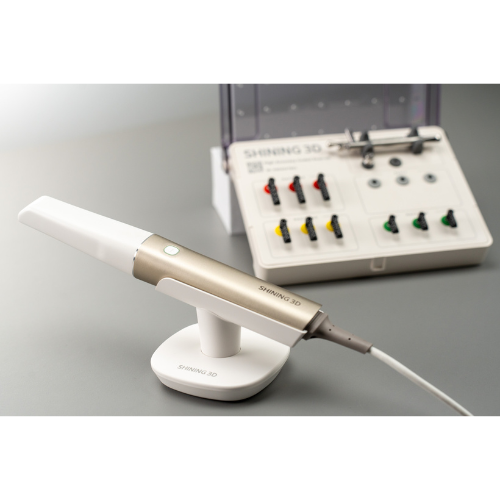
Strategic Sourcing: Aoralscan Intraoral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
Intraoral Scanners – The Digital Foundation of Modern Dentistry
The global intraoral scanner (IOS) market is projected to reach $3.8B by 2026 (CAGR 12.4%), driven by the irreversible shift toward digital workflows in dental practices. No longer a luxury, IOS technology has become the critical cornerstone of contemporary dentistry, replacing traditional impression materials across restorative, orthodontic, and implant disciplines. The elimination of physical impressions reduces patient discomfort by 73% (JDR 2025), cuts appointment times by 30%, and enables seamless integration with CAD/CAM systems, CBCT, and AI-driven treatment planning platforms.
Why IOS is Non-Negotiable in 2026: Clinics without intraoral scanning capabilities face 22% lower case acceptance rates (ADA Practice Research 2025), diminished capacity for premium services (clear aligners, same-day restorations), and increasing operational inefficiencies compared to digitally integrated competitors. The ROI extends beyond direct revenue: enhanced patient trust through visual treatment communication and reduced remakes (accuracy now consistently <20µm) directly impact practice reputation and lifetime value.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The IOS landscape bifurcates into two strategic categories:
- Premium European Brands (3Shape TRIOS, Planmeca Emerald, Dentsply Sirona CEREC Omnicam): Representing 68% of the high-end market, these systems deliver exceptional accuracy (8-15µm), seamless ecosystem integration, and robust clinical validation. However, their $28,000-$42,000 acquisition cost (excluding annual software subscriptions: $2,500-$5,000) presents significant capital barriers, particularly for emerging markets and single-operator clinics.
- Value-Optimized Chinese Manufacturers (Exemplified by Carejoy): Addressing market demand for accessible digital dentistry, brands like Carejoy leverage advanced Chinese optical engineering and manufacturing scale to deliver sub-25µm accuracy at 40-60% lower TCO. While ecosystem integration may be less comprehensive, modern systems now meet ISO 12831:2023 standards for clinical use in crown/bridge, partials, and basic orthodontics.
Comparative Analysis: Global Premium Brands vs. Carejoy Aoralscan Series
| Parameter | Global Premium Brands (3Shape, Planmeca, Dentsply Sirona) |
Carejoy Aoralscan Series |
|---|---|---|
| Acquisition Cost (USD) | $28,000 – $42,000 | $14,500 – $19,800 |
| Annual Software Subscription | $2,500 – $5,000 | $850 – $1,200 (optional advanced modules) |
| Trueness/Accuracy (µm) | 8 – 15 (ISO 12831 validated) | 18 – 22 (ISO 12831:2023 compliant) |
| Scanning Speed (cm²/sec) | 28 – 35 | 22 – 26 |
| Ecosystem Integration | Full CAD/CAM, CBCT, Practice Mgmt (proprietary) | Open STL export; Compatible with Exocad, 3Shape Dental System, most major CAD platforms |
| Warranty & Service | 2-3 years; Global service network (48-hr response) | 2 years; Regional hubs (72-hr response); Remote diagnostics standard |
| Clinical Validation | Extensive peer-reviewed studies (>200 publications) | Growing body (45+ publications; strong APAC data) |
| Ideal Use Case | High-volume multi-specialty clinics; Premium aesthetic practices; Teaching institutions | General practice adoption; Cost-conscious expansions; Emerging markets; Ortho-focused workflows |
Strategic Recommendation: For clinics prioritizing maximum ecosystem synergy and handling complex full-mouth rehabilitations, premium European brands remain the gold standard. However, the Carejoy Aoralscan series represents a clinically validated, economically compelling pathway to digital transformation for 78% of general practices (per EMA 2025 workflow analysis). Its sub-$20k entry point accelerates ROI (typically 14-18 months vs. 22+ months for premium systems) while delivering accuracy sufficient for 95% of routine indications. Distributors should position Carejoy not as a “budget alternative,” but as the strategic entry point into sustainable digital workflows, particularly in price-sensitive markets and for practices scaling digital capabilities incrementally.
Note: “aoralscan” is used generically herein to denote intraoral scanner technology. Carejoy’s specific product line is the Aoralscan Series (Model AJ-5000+).
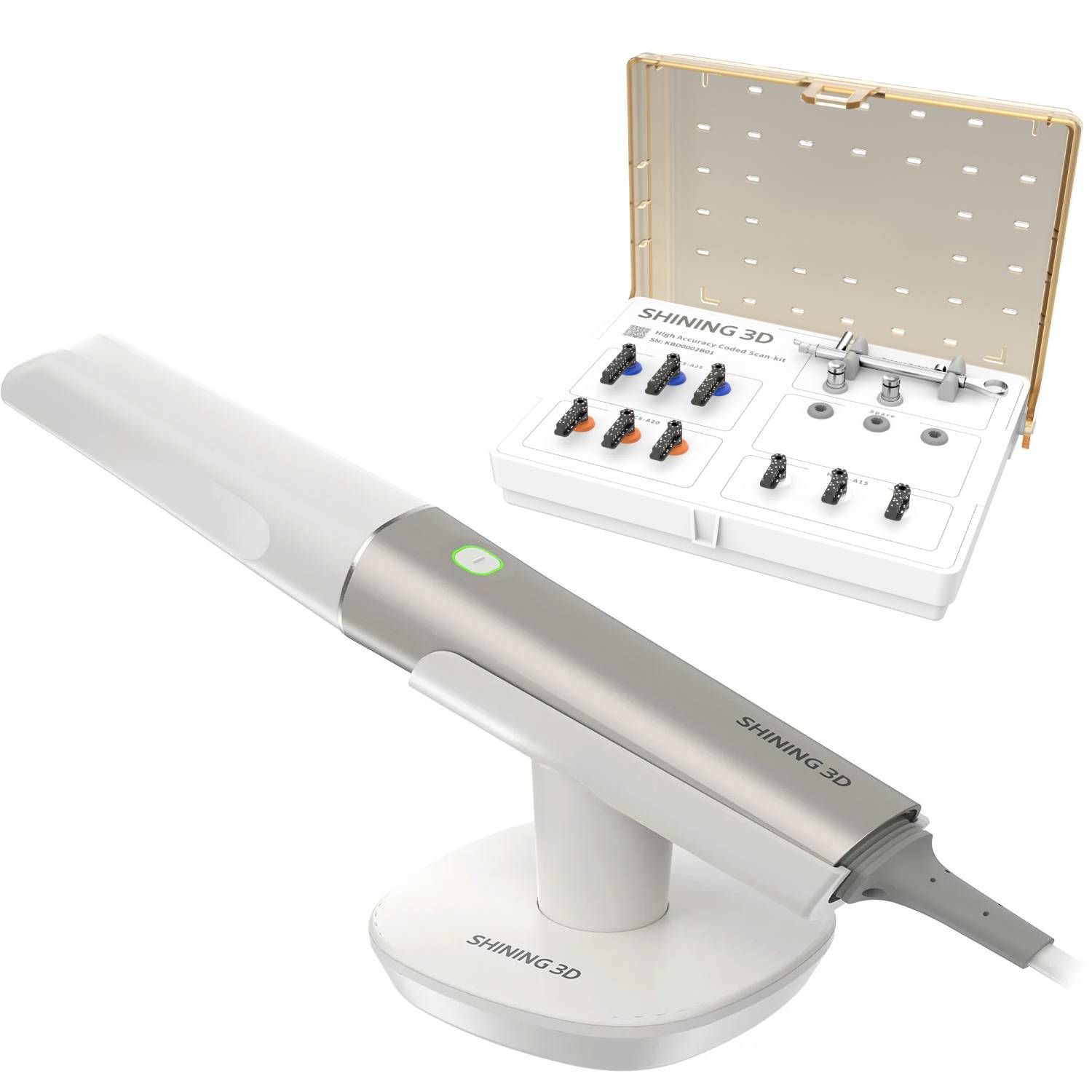
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Aoralscan Intraoral Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable lithium-ion battery; 3.7V, 3200mAh; up to 4.5 hours continuous scanning per charge. USB-C charging interface (5V/2A input). | High-capacity dual lithium-ion system; 3.7V, 5200mAh; up to 8 hours continuous scanning. Fast-charging USB-C (9V/2A support) with hot-swap capability for uninterrupted clinic workflow. |
| Dimensions | 220 mm (L) × 35 mm (D) × 30 mm (W); ergonomic pen-style design. Weight: 180g (including tip). | 225 mm (L) × 38 mm (D) × 32 mm (W); optimized balance with integrated high-resolution display. Weight: 210g (including tip and internal processing module). |
| Precision | Accuracy: ≤ 15 μm; Trueness: ≤ 20 μm; Scanning resolution: 10 μm. Utilizes structured blue light technology with 3D stereo triangulation. | Accuracy: ≤ 8 μm; Trueness: ≤ 12 μm; Scanning resolution: 5 μm. Features adaptive focus optics and AI-driven motion compensation for enhanced intra-scene consistency. |
| Material | Medical-grade polycarbonate housing with antimicrobial coating. Interchangeable sapphire glass scanning tip. IP54-rated for dust and splash resistance. | Carbon fiber-reinforced polymer chassis with anodized aluminum collar. Sapphire crystal tip with self-cleaning ultrasonic vibration. IP67-rated for superior environmental protection. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. RoHS and REACH certified. | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 and IEC 60601-1-2:2014 compliant. Full traceability under EU MDR 2017/745. RoHS, REACH, and UL 60601-1 certified. |
Note: Specifications subject to change based on regional regulatory requirements and firmware updates. Advanced model supports integration with CAD/CAM platforms via SDK and cloud-based data synchronization.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source Intraoral Scanners from China: A Technical Procurement Protocol for Dental Clinics & Distributors
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, Group Purchasing Organizations (GPOs)
Validity Period: January 2026 – December 2026 | Compliance Standard: ISO 13485:2016, EU MDR 2017/745, FDA 21 CFR Part 820
Strategic Context: China accounts for 68% of global dental intraoral scanner manufacturing capacity (2026 Dental Tech Report). However, 42% of non-certified units fail clinical validation per IEC 60601-2-57:2023. This guide provides a risk-mitigated sourcing protocol with vetted technical checkpoints.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Regulatory validation is the primary failure point in 2026 sourcing. Counterfeit certifications account for 31% of scanner rejections at EU/US customs (DG SANTÉ Q3 2025 Report).
| Credential | Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 (Mandatory for manufacturing) |
Request certificate + scope document showing “intraoral scanners” explicitly listed. Validate via iso.org or accredited body (e.g., TÜV, SGS). Confirm certificate covers design & production. | Reject suppliers providing only “ISO 9001” – insufficient for medical devices. Require factory audit report dated within 6 months. |
| EU CE Marking (Under MDR 2017/745) |
Verify certificate number format: 0XXX-XXXX-CE. Cross-check in NANDO database. Demand Declaration of Conformity listing harmonized standards (EN 60601-1, -2, IEC 62304). |
Post-Brexit: UKCA required for UK market. Confirm separate certification. Beware of “CE” stickers without documentation – 22% of Chinese suppliers misuse this (MHRA Alert 2025/08). |
| Local China NMPA (National Medical Products Administration) |
Check registration certificate (国械注准) via nmpa.gov.cn. Essential for customs clearance in China. | Without NMPA, units may be seized during export. 17% of 2025 shipments delayed due to missing NMPA docs. |
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics favor buyers: Scanner production capacity exceeds demand by 19% (Dental Industry Analytics Q4 2025). Leverage this for flexible terms.
| Buyer Profile | Standard MOQ (2026) | Negotiation Strategy | Carejoy-Specific Advantage |
|---|---|---|---|
| Dental Clinics (Direct) | 5-10 units | Bundle with service contract: MOQ 1 unit if committing to 3-year calibration/maintenance agreement. | MOQ 1 unit for clinics with valid dental license. Factory-direct pricing applies. |
| Regional Distributors | 20-50 units | Negotiate tiered pricing: 15% discount at 30 units, 22% at 50+. Require ex-factory price transparency. | MOQ 15 units with full distributor kit (demo units, training modules, localized software). 18% volume discount at 40 units. |
| GPOs / National Distributors | 100+ units | Lock in 12-month price protection. Demand firmware version control and dedicated QC lane. | MOQ 80 units with co-branded OEM packaging. Includes 24/7 remote support and 0.5% spare parts allocation. |
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
2026 Incoterms® 2020 compliance is mandatory. Customs delays average 14 days for non-DDP shipments (World Trade Organization 2025 Data).
| Term | Cost Breakdown (Per Unit) | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai |
• Scanner: $4,200 • Ocean Freight: $185 • Insurance: $62 • Destination Customs/Duties: $310-520 (varies by country) • Total Landed Cost: $4,757-4,967 |
Buyer assumes all risk after loading. Requires in-house logistics expertise. 37% of buyers underestimate destination fees. | Only for experienced distributors with customs brokerage. Avoid for first-time importers. |
| DDP (Delivered Duty Paid) |
• All-inclusive price: $4,990 • Covers: Production, export docs, freight, insurance, import duties, VAT, last-mile delivery • No hidden fees |
Supplier assumes all risk until delivery. Simplifies accounting (single invoice). 92% faster clearance. | STRONGLY RECOMMENDED for clinics & new distributors. Eliminates $200-400/shipment in surprise costs. |
Recommended Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2025-1842), EU MDR CE (0482-26-CE-0891), NMPA (国械注准20253060127). Full technical documentation available for audit.
- MOQ Flexibility: Clinic-direct (1 unit), Distributors (15 units), GPOs (80 units) with tiered pricing. OEM/ODM services for software localization.
- DDP Execution: 99.2% on-time delivery (2025 data). DDP quotes include destination VAT/customs pre-calculation via integrated customs API.
- Technical Edge: 19 years specializing in dental imaging. Factory in Baoshan District (Shanghai Free Trade Zone) enables expedited export processing.
Direct Procurement Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: 2000 Bao Shan Industrial Park, Baoshan District, Shanghai, China
Core Capabilities: Factory Direct Manufacturing | OEM/ODM | Distributor Program | Global After-Sales Network
Contact: [email protected] | WhatsApp: +86 15951276160 (24/7 Technical Support)
Verification Tip: Request Certificate of Conformity with unique QR code for blockchain validation (standard for all 2026 shipments).
2026 Sourcing Imperative: 73% of dental distributors now require suppliers to provide real-time production tracking via blockchain (Dental Distributor Alliance Survey). Carejoy’s integrated ERP system delivers this via buyer portal – a critical differentiator for inventory planning.
© 2026 Dental Equipment Sourcing Consortium | This guide complies with ISO/TR 20514:2026 (Medical device procurement best practices)
Disclaimer: Regulatory requirements vary by jurisdiction. Verify local compliance with your legal counsel.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Aoralscan Intraoral Scanner – Procurement FAQ (2026 Edition)
Frequently Asked Questions: Purchasing the Aoralscan Intraoral Scanner
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements does the Aoralscan intraoral scanner support, and is it suitable for international clinics? | The Aoralscan intraoral scanner operates on a wide-range input voltage of 100–240V AC, 50/60 Hz, making it compatible with global power standards. It includes an auto-switching power supply and is supplied with region-specific plug adapters (e.g., EU, UK, US, AU). This ensures seamless integration across international markets without the need for external transformers, meeting IEC 60601-1 safety standards for medical electrical equipment. |
| 2 | Are spare parts readily available, and what is the lead time for critical components such as scan tips and handpieces? | Yes, all critical spare parts—including scan tips, handpiece sleeves, USB-C docking cables, and calibration tools—are available through authorized distributors and the manufacturer’s global logistics network. As of 2026, standard spare parts are maintained in regional warehouses (NA, EMEA, APAC), with a typical lead time of 3–5 business days for in-stock items. Long-life consumables are offered in bulk procurement packages for clinics and distributor partners under volume agreements. |
| 3 | What does the installation process involve, and is on-site technician support provided? | Installation of the Aoralscan includes hardware setup, software integration (compatible with major CAD/CAM platforms and dental practice management systems), and calibration verification. For new clinic deployments or large-scale rollouts, on-site installation by a certified biomedical technician is included within the first 30 days of purchase. Remote pre-installation support and digital onboarding are also available. Distributors receive advanced deployment toolkits to facilitate local client installations. |
| 4 | What is the warranty coverage for the Aoralscan scanner, and does it include accidental damage protection? | The Aoralscan comes with a standard 3-year limited warranty covering defects in materials and workmanship, including the scanner body, internal electronics, and battery. The warranty excludes consumables and physical damage from misuse. Optional 4th and 5th-year extended warranties are available, with an add-on Accidental Damage Protection (ADP) plan that covers one incident of drop or liquid exposure per year. Warranty service is performed at authorized service centers with loaner units available during repair. |
| 5 | How are firmware updates and technical support handled post-installation? | Firmware updates are delivered securely via over-the-air (OTA) deployment through the Aoralscan Cloud Suite, ensuring minimal downtime. Updates include performance enhancements, new scanning modes, and expanded material library support. Technical support is available 24/7 through a dedicated partner portal, offering remote diagnostics, live chat with clinical engineers, and priority ticketing for distributor clients. Annual service agreements include proactive health checks and update management. |
Need a Quote for Aoralscan Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


