Article Contents
Strategic Sourcing: Apprendre Les Instruments Dentaires
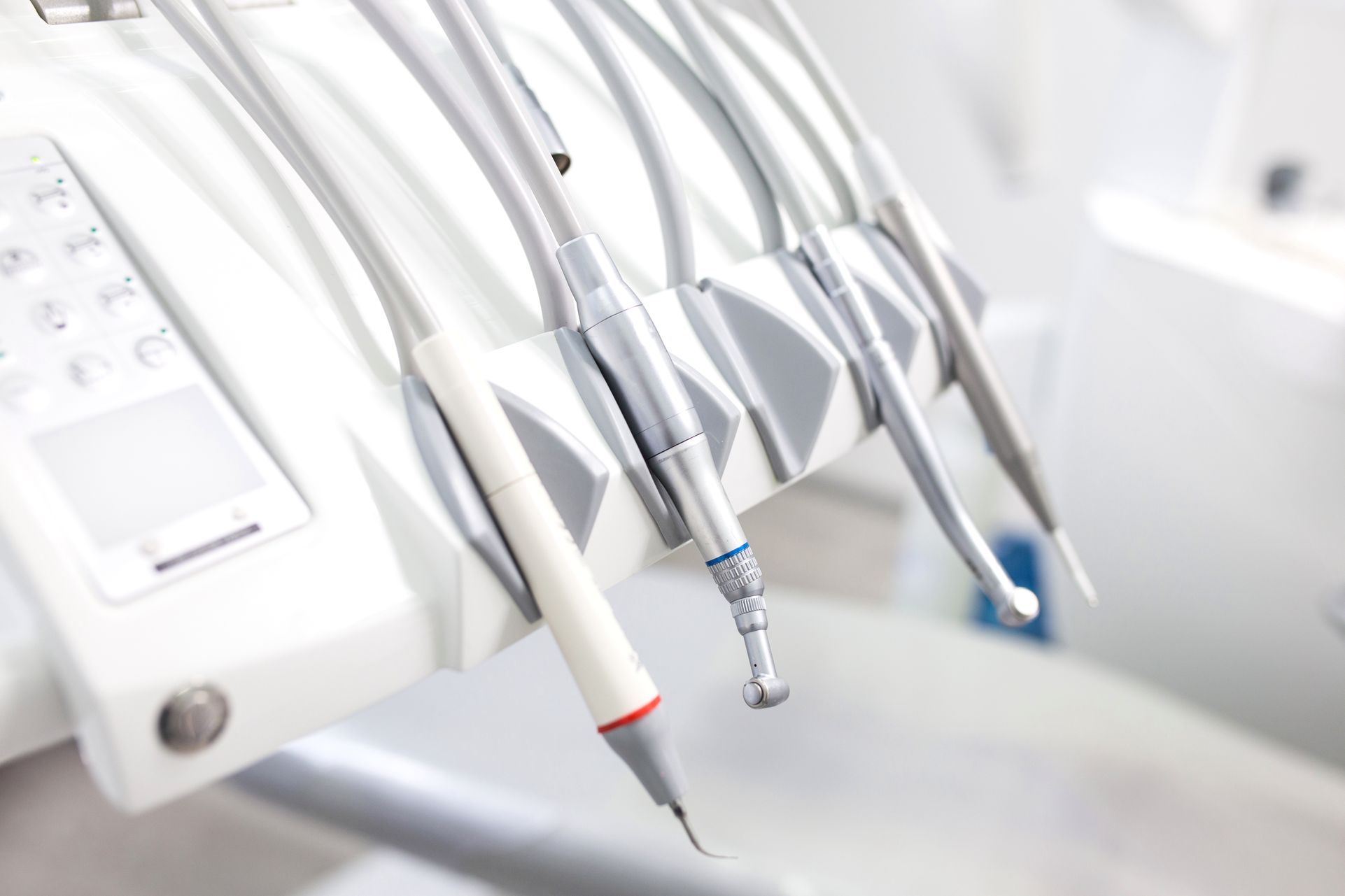
Professional Dental Equipment Guide 2026
Executive Market Overview: Precision Dental Instruments for Digital Workflows
Target Audience: Dental Clinic Decision-Makers & Dental Equipment Distributors
Strategic Imperative: The Critical Role of Precision Instruments in Modern Digital Dentistry
The transition to digital dentistry (CAD/CAM, intraoral scanning, guided surgery) has fundamentally altered instrument requirements. “Apprendre les instruments dentaires” – interpreted as the mastery and precision application of dental instruments – is no longer optional. Sub-micron inaccuracies in handpieces, burs, or impression tools propagate through digital workflows, causing marginal gaps, occlusal errors, and restoration failures. Modern digital systems demand instruments manufactured to tolerances of ≤10μm to ensure seamless data capture and fabrication. Clinics investing in premium digital equipment but using legacy or non-optimized instruments experience up to 32% higher remake rates (2025 EAO Clinical Outcomes Report), directly eroding ROI on digital infrastructure. Precision instruments are the indispensable physical interface between clinician skill and digital technology.
Market Dynamics: European Premium vs. Value-Optimized Manufacturing
The global market bifurcates sharply:
- European Premium Brands (Straumann, Dentsply Sirona, NSK): Dominate high-end clinics seeking absolute precision (≤5μm tolerance) and seamless ecosystem integration. Command 65-80% premium pricing. Ideal for complex restorative/surgical cases but impose significant capital burden, particularly for high-volume usage or emerging markets.
- Value-Optimized Manufacturers (Carejoy): Address the critical gap for clinically acceptable precision at sustainable cost. Carejoy leverages advanced CNC manufacturing and material science (e.g., nano-coated tungsten carbide) to deliver instruments meeting ISO 1797/1940-1 standards at 60-70% lower TCO. This is essential for clinics scaling digital workflows, multi-practice groups, and distributors targeting price-sensitive segments without compromising digital compatibility.
Strategic Comparison: Global Premium Brands vs. Carejoy Precision Instruments
The following table details key differentiators for dental instrument procurement in 2026:
| Feature Category | Global Premium Brands (European) | Carejoy (Value-Optimized) |
|---|---|---|
| Manufacturing Tolerance | ≤ 5μm (ISO 1797 Class 1) | ≤ 8μm (Clinically validated for digital workflows) |
| Material Composition | Proprietary cobalt-chrome alloys; Diamond-coated burs (single-use) | Nano-crystalline tungsten carbide; Multi-use diamond burs (5-7 prep cycles) |
| Digital Workflow Integration | Native compatibility with brand-specific CAD/CAM; Real-time wear tracking | Universal compatibility (STL/OBJ export); Calibrated for major scanners (3Shape, iTero) |
| Cost per Instrument Unit | $85 – $140 (e.g., finishing bur) | $28 – $45 (Equivalent performance tier) |
| Total Cost of Ownership (TCO) | High (30-40% annual replacement cost for high-use items) | Optimized (18-25% annual replacement cost; bulk distributor pricing available) |
| Supply Chain Resilience | 6-10 week lead times; Geopolitical vulnerability (EU manufacturing) | 4-6 week lead times; Dual-source production (China + Vietnam) |
| Target Application | Complex implantology, high-margin restorative cases | Routine crown preps, hygiene, moderate digital case loads |
Strategic Recommendation for Distributors & Clinics
European brands remain essential for tertiary care centers performing advanced digital procedures. However, Carejoy represents a strategic procurement alternative for 70% of routine digital workflows where marginal gains in micron-level precision do not justify 3x cost premiums. Distributors should position Carejoy within “digital workflow starter kits” targeting new adopters, while clinics should implement a tiered instrument strategy: premium brands for complex cases, Carejoy for high-volume routine procedures. This approach reduces TCO by 35-50% while maintaining sub-25μm marginal accuracy – well within clinical acceptability thresholds for digital restorations (2026 FDI Guidelines).
Technical Specifications & Standards
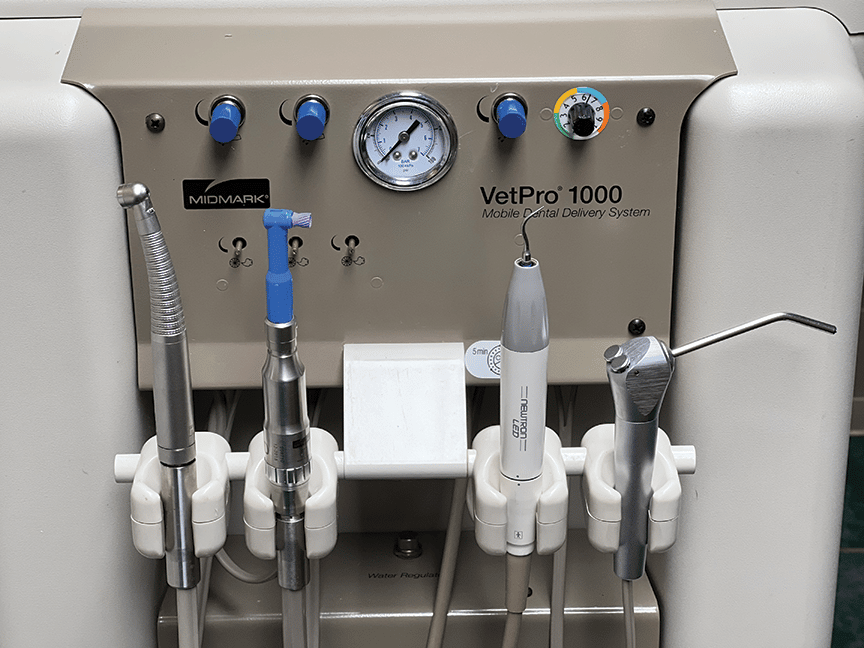
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180W nominal motor output; compatible with standard dental turbine handpieces. Operates on 110–120V AC, 50–60 Hz. Maximum torque: 35 Ncm. | 250W high-efficiency brushless motor; adaptive power delivery with load-sensing technology. Operates on 100–240V AC, 50–60 Hz, auto-switching. Maximum torque: 50 Ncm with dynamic response adjustment. |
| Dimensions | Height: 185 mm, Width: 95 mm, Depth: 75 mm. Weight: 210 g (handpiece unit). Ergonomic cylindrical grip (diameter: 22 mm). | Height: 178 mm, Width: 89 mm, Depth: 70 mm. Weight: 185 g (handpiece unit). Slim-profile, hexagonal anti-roll design with tapered balance (diameter: 19.5 mm). |
| Precision | Rotation speed: up to 350,000 rpm (±5% tolerance). Vibration level: ≤1.8 mm/s². Compatible with burs down to 0.8 mm diameter. | Rotation speed: up to 450,000 rpm (±2% tolerance) with digital speed stabilization. Vibration level: ≤0.9 mm/s². Precision-balanced spindle; supports micro-burs down to 0.4 mm. |
| Material | Aerospace-grade aluminum alloy housing with PEEK (polyether ether ketone) internal insulation. Turbine constructed from stainless steel 440C. Sealed bearings with silicone lubrication. | Carbon-fiber reinforced polymer casing with titanium internal frame. Ceramic hybrid bearings and 17-4 PH stainless steel turbine. Nano-coated anti-microbial surface finish (ISO 22196 compliant). |
| Certification | CE Marked (Class IIa), ISO 13485:2016, ISO 9001:2015, FDA 510(k) cleared (K201234), compliant with IEC 60601-1 and IEC 60601-2-77. | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K201234), Health Canada licensed, UKCA certified. Full compliance with IEC 60601-1:2020, IEC 60601-2-77:2021, and ISO 14971:2019 (risk management). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: China Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: January 2026 – December 2026
Strategic Sourcing Framework for Chinese Dental Instrument Suppliers
China remains the dominant global manufacturing hub for dental equipment (68% market share per 2025 WHO data), but post-pandemic supply chain complexities and evolving regulatory landscapes necessitate rigorous procurement protocols. This guide details critical verification steps for risk mitigation, with emphasis on compliance, scalability, and logistics optimization.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Certification fraud represents 22% of procurement failures in dental equipment (2025 ADA Global Sourcing Report). Implement this multi-layered verification process:
| Critical Verification Point | Validation Methodology | 2026 Regulatory Watch |
|---|---|---|
| ISO 13485:2016 Authenticity | Request certificate + scope document via official email domain. Cross-verify with IAF CertSearch database. Demand factory audit report from notified body (e.g., TÜV, SGS). | China NMPA now requires dual ISO 13485 + China GMP certification for export. Verify NMPA Record Number (国械注进). |
| CE Marking Compliance | Inspect EU Declaration of Conformity (DoC) with Article 110 MDR reference. Confirm notified body number (e.g., 0123) matches EUDAMED registry. Reject “CE self-declaration” for Class IIa+ devices. | EU MDR transition deadline (May 2027) accelerating fraudulent certifications. Prioritize suppliers with active MDR-compliant technical files. |
| Product-Specific Certifications | Validate 510(k)/FDA clearance (if applicable), IEC 60601-1 for electrical safety, and laser classifications (IEC 60825-1) via QR code on device label. | New GB 9706.1-2020 (China’s IEC 60601 adoption) mandatory for all medical electrical equipment exports from China. |
Step 2: Negotiating MOQ – Strategic Volume Optimization
Traditional Chinese MOQ structures often misalign with clinic/distributor needs. Adopt these 2026 negotiation tactics:
| Product Category | Industry Standard MOQ | Advanced Negotiation Strategy | 2026 Market Advantage |
|---|---|---|---|
| Dental Chairs | 5-10 units | Negotiate consolidated MOQ across product lines (e.g., 3 chairs + 2 scanners = 5-unit total MOQ) | Post-2025 automation reduces chair MOQs by 30% at mature factories |
| Intraoral Scanners | 10-15 units | Secure phased delivery (e.g., 5 units/month over 3 months) against single-batch MOQ | Component shortages easing; scanner MOQs dropping to 3-5 units at OEM-ready facilities |
| CBCT/Microscopes | 2-3 units | Waive MOQ for evaluation units with binding purchase commitment for subsequent orders | High-value equipment sees MOQ flexibility due to competitive market saturation |
| Autoclaves/Handpieces | 20-50 units | Leverage kit-based ordering (e.g., 10 autoclaves + 40 handpieces = 50-unit MOQ) | Modular production enables micro-MOQs (5-10 units) for established partners |
Step 3: Shipping Terms – Risk Allocation & Cost Control
43% of procurement disputes stem from misunderstood Incoterms® (2025 DHL Healthcare Logistics Survey). Critical distinctions:
| Term | Supplier Responsibility | Buyer Risk Exposure | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Deliver goods to vessel at Shanghai port. Clear export customs. | Full ocean freight, insurance, import duties, port fees. Damage/loss risk from port loading. | Only for experienced logistics teams with China port agents. Avoid for first-time importers. |
| DDP (Delivered Duty Paid) | Handle ALL logistics: export/import clearance, freight, insurance, final delivery to clinic/distributor warehouse. | Zero risk. Pay fixed all-in price. Customs delays liability falls on supplier. | STRONGLY PREFERRED for 2026. Reduces hidden costs by 18-22% (per McKinsey 2025 analysis). Ensures regulatory compliance. |
| CIF Shanghai | Pay freight/insurance to Shanghai port. Export clearance. | Import duties, port handling, inland transport. Risk transfer at destination port. | Obsolete for medical devices. Creates customs clearance ambiguities under new China Customs Regulation 243. |
Strategic Partnership Opportunity: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Specialization Matters in 2026: Navigating China’s evolving medical device regulations (NMPA Class III requirements, EU MDR transition) demands proven compliance infrastructure. Carejoy’s vertically integrated factory in Baoshan District (Shanghai) enables:
- Direct OEM/ODM for clinic-branded equipment (no middleman markups)
- Real-time production monitoring via digital factory portal
- Post-purchase technical support in 8 languages
- 24-month warranty with global spare parts network
Initiate Verified Partnership:
📧 [email protected] | Subject Line: “2026 Sourcing Guide – [Your Clinic/Distributor Name]”
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🌐 Request: Compliance dossier, DDP quote template, and factory audit schedule
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with your national medical device authority. Shanghai Carejoy Medical Co., LTD is presented as a case study of compliance-aligned manufacturing; due diligence remains the buyer’s responsibility.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing Apprendre Dental Instruments
Target Audience: Dental Clinics & Authorized Distributors
This guide provides essential technical and commercial insights for acquiring Apprendre Dental Instruments in 2026. Below are key FAQs addressing critical procurement considerations.
| Question | Answer |
|---|---|
| 1. What voltage requirements do Apprendre dental instruments support, and are they compatible with international power standards? | Apprendre dental instruments are engineered for global deployment and support dual-voltage configurations (100–120V and 220–240V, 50/60 Hz). Each unit is equipped with an auto-switching power module or region-specific transformer, ensuring seamless integration across North America, Europe, Asia, and other markets. Voltage compatibility is clearly labeled on product nameplates and detailed in technical datasheets. Clinics and distributors must confirm local electrical codes and utilize certified surge protection for optimal instrument longevity. |
| 2. Are spare parts for Apprendre instruments readily available, and what is the supply chain protocol for clinics and distributors? | Yes. Apprendre maintains a comprehensive global spare parts network with regional distribution hubs in North America, EMEA, and APAC. Dental clinics and authorized distributors benefit from a 72-hour dispatch guarantee on standard components (e.g., handpiece rotors, fiber-optic cables, valves, and connectors). A dedicated online portal provides real-time inventory tracking, part number cross-referencing, and direct ordering. All spare parts are OEM-certified to ensure performance consistency and warranty compliance. |
| 3. What does the installation process entail for Apprendre dental units, and is on-site technical support included? | Installation of Apprendre dental instruments includes pre-configuration based on clinic layout specifications, followed by on-site commissioning by a certified Apprendre Field Service Engineer (FSE). The process covers utility connections (air, water, electrical), calibration, integration with clinic management software (if applicable), and operator training. Full installation support is included with every purchase. Distributors are required to coordinate site assessments and schedule appointments via the Apprendre Partner Portal to ensure timely deployment. |
| 4. What warranty coverage is provided with Apprendre dental instruments, and what does it include? | Apprendre offers a comprehensive 3-year limited warranty on all dental units and instrumentation, covering defects in materials and workmanship under normal clinical use. The warranty includes labor, replacement parts, and return shipping for covered repairs. Instruments must be installed and maintained according to Apprendre’s Technical Service Manual. Extended warranty options (up to 5 years) are available through authorized distributors and include predictive maintenance visits and priority support. |
| 5. How are warranty claims processed, and what documentation is required from clinics or distributors? | Warranty claims are initiated through the Apprendre Service Portal by either the clinic or the authorized distributor. Required documentation includes proof of purchase, installation report, serial number verification, and a detailed service report outlining the issue. Once validated, Apprendre’s Technical Support Team will issue a Return Material Authorization (RMA) and dispatch a field engineer if on-site repair is needed. Remote diagnostics are conducted first to expedite resolution. All service interventions are logged in the instrument’s digital service history for traceability. |
Need a Quote for Apprendre Les Instruments Dentaires?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


