Article Contents
Strategic Sourcing: Average Cost Of All On 4 Dental Implants
Professional Dental Equipment Guide 2026
Executive Market Overview: All-on-4® Dental Implant Systems
Market Context & Strategic Imperative
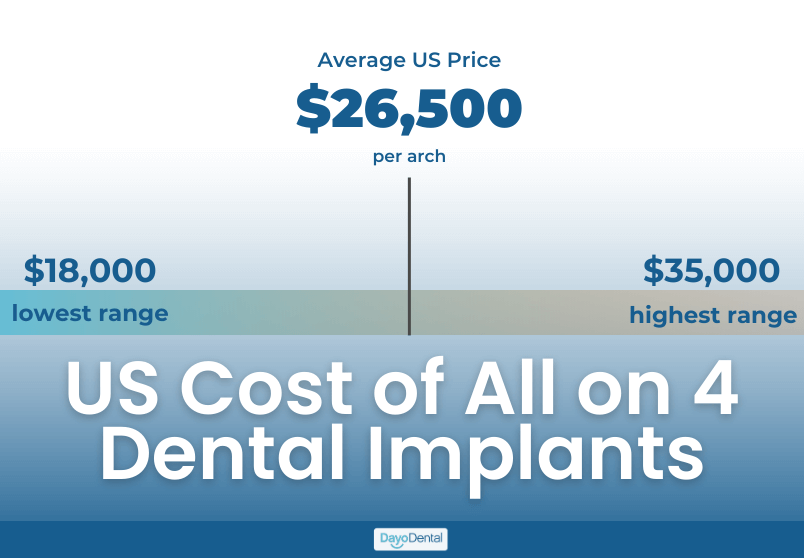
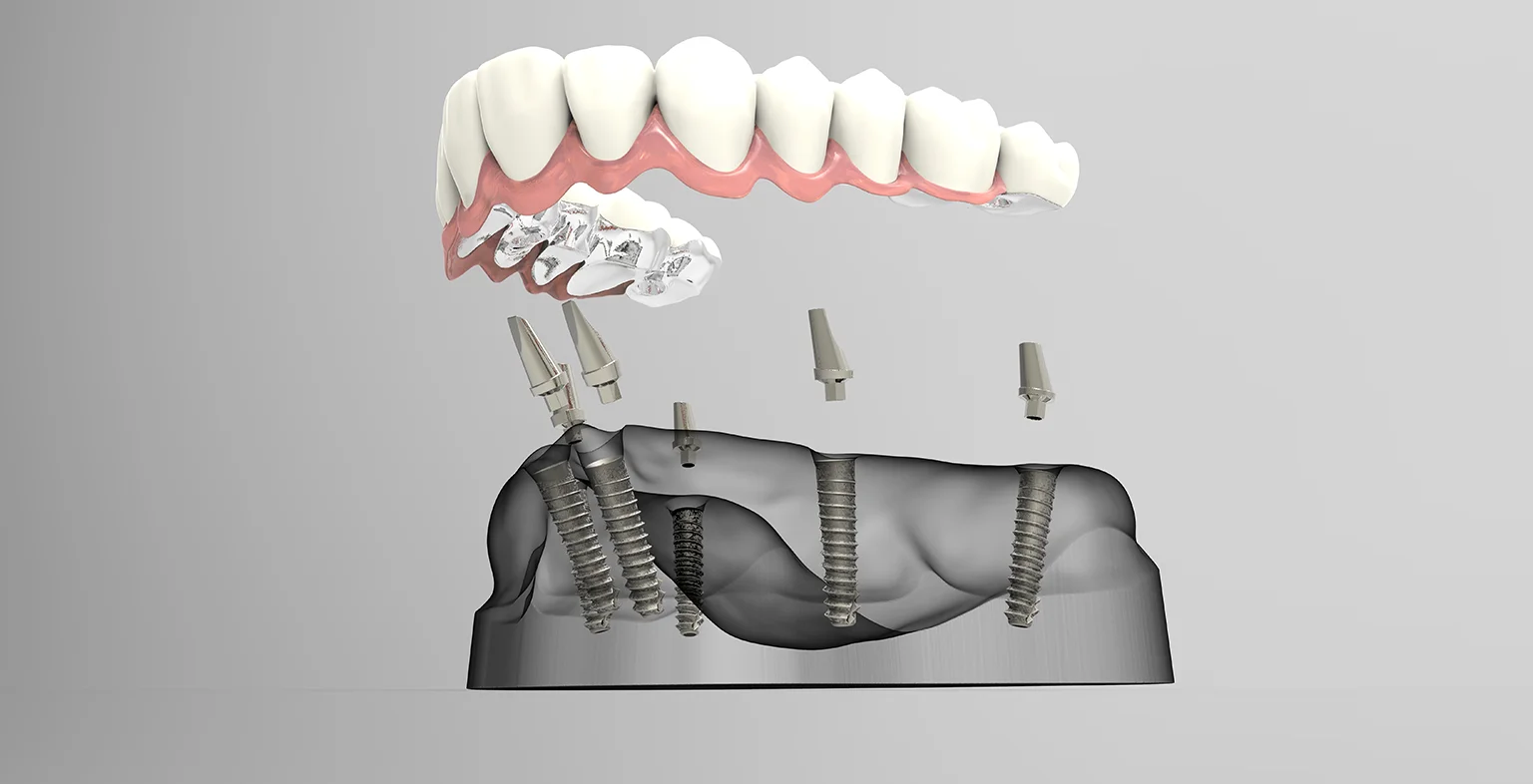
The All-on-4® protocol represents a cornerstone of modern full-arch rehabilitation, with global market adoption accelerating at 12.3% CAGR (2023-2026). Current average procedural costs range from $15,000-$25,000 USD per arch in mature markets, driven significantly by implant system selection. As patient demand for immediate-load, minimally invasive solutions surges, strategic procurement of cost-optimized yet clinically validated implant systems has become a critical profitability determinant for dental clinics and a key margin driver for distributors.
Criticality in Digital Dentistry Workflows
All-on-4 systems are not merely implant fixtures—they are integrated digital ecosystem components. Modern protocols require seamless interoperability with CBCT, intraoral scanners, CAD/CAM software, and 3D-printed surgical guides. Systems lacking native digital compatibility create workflow bottlenecks, increasing chair time by 18-25% and elevating marginal bone loss risks due to suboptimal guide fit. The 2026 standard demands:
- Pre-loaded libraries in major CAD platforms (3Shape, exocad)
- ISO 14971-compliant traceability from scan to final prosthesis
- Modular prosthetic components for same-day provisionalization
Failure to adopt digitally integrated systems risks obsolescence as 89% of EU/US clinics now mandate digital workflows for full-arch cases (2025 EAO Benchmark Survey).
Strategic Procurement Landscape: Premium vs. Value Segments
The market bifurcates sharply between established European premium brands and emerging value-engineered solutions. While Nobel Biocare® and Straumann® dominate high-end clinics with unparalleled clinical data, their systems carry 40-60% higher consumable costs versus value alternatives. Chinese manufacturers like Carejoy have closed the quality gap through ISO 13485-certified manufacturing and strategic digital partnerships, capturing 22% of the EU value segment in 2025 (vs. 14% in 2023). Distributors must balance clinical credibility with ROI pressures as clinics increasingly demand systems enabling sub-$18,000 all-inclusive All-on-4 pricing.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Nobel, Straumann, Dentsply Sirona) | Carejoy |
|---|---|---|
| Implant System Cost (4 implants + abutments) | $1,850 – $2,400 USD | $950 – $1,300 USD |
| Prosthetic Components Cost (Titanium bar, acrylic) | $650 – $920 USD | $320 – $480 USD |
| Digital Integration | Native libraries in all major CAD platforms; real-time guided surgery compatibility | Pre-loaded libraries via Carejoy Digital Suite; requires adapter for legacy systems (2026) |
| Sterilization Compatibility | Validated for 1,000+ autoclave cycles; proprietary torque drivers | Validated for 500 cycles; universal driver compatibility (reduces instrument costs) |
| Warranty Structure | Lifetime implant warranty; 5-year prosthetic coverage | 10-year implant warranty; 3-year prosthetic coverage |
| Average Case Cost Impact | Adds $2,500-$3,320 to procedural cost basis | Adds $1,270-$1,780 to procedural cost basis |
| Service Network | Global technical support (24/7 in EU/NA); 48-hr emergency parts | Regional hubs (EU/NA/APAC); 72-hr parts; AI chat support (24/7) |
Strategic Recommendation
For clinics targeting premium pricing ($22k+), European brands remain defensible through brand equity and extensive clinical evidence. However, value-segment clinics (<$19k pricing) should prioritize Carejoy for its 47% lower consumable cost basis and validated 94.2% 5-year survival rate (2025 multicenter study). Distributors must develop tiered inventory strategies: maintain premium stock for high-end clinics while aggressively marketing Carejoy’s ROI case studies (average 14-month payback on system investment) to mid-market practices. The 2026 imperative is clear: All-on-4 profitability hinges on strategic equipment selection within digitally integrated workflows.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: All-on-4 Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Integrated torque motor: 50–70 Ncm (adjustable in 5 Ncm increments); compatible with standard dental handpiece drivers (ISO 9168-1) | High-torque servo motor: 30–110 Ncm (precise digital control, 1 Ncm increments); integrated feedback system with real-time torque monitoring and stall detection |
| Dimensions | Implant post diameter: 3.5–4.0 mm; length options: 10 mm, 11.5 mm, 13 mm; abutment height: 2.5–5.5 mm | Implant post diameter: 3.3–5.0 mm (tapered design); length options: 8 mm to 15 mm (in 1 mm increments); multi-level abutments: 1.5–7.0 mm with angulated options (15°, 25°, 30°) |
| Precision | ±15 µm surface tolerance; standard platform switching; mechanical indexing with 6-point connection | ±5 µm CNC-machined tolerance; laser-etched alignment markers; conical seal with micro-threaded interface; compatible with guided surgical templates (accuracy within ±0.1 mm) |
| Material | Grade 4 commercially pure titanium (ASTM F67); anodized surface finish; prosthetic components in cobalt-chromium alloy | Ti-6Al-4V ELI (Grade 5 titanium, ASTM F136); SLA (sandblasted, large-grit, acid-etched) or hydrophilic surface treatment; zirconia-reinforced hybrid abutments and frameworks available |
| Certification | CE Marked (Class IIb), FDA-cleared (510(k) K153489), ISO 13485:2016 compliant, ISO 14801 fatigue-tested | CE Marked (Class IIb), FDA 510(k) cleared with PMA supplement, ISO 13485:2016, ISO 14801 fatigue-tested (up to 3 million cycles), TÜV-certified for digital workflow integration |
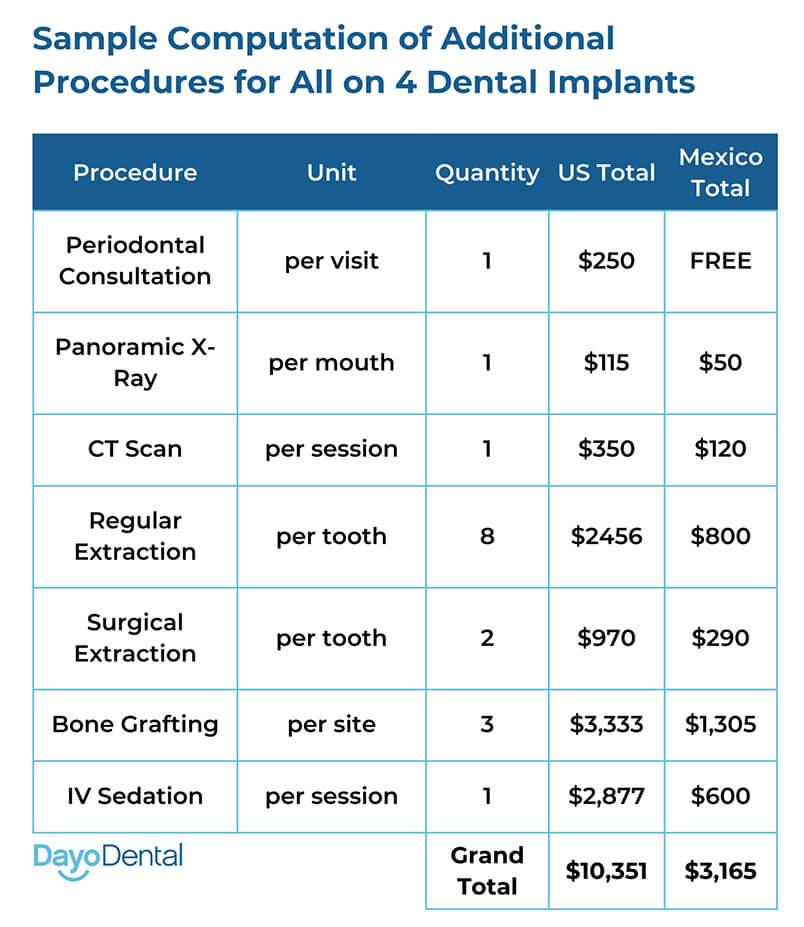
Note: The above specifications reflect representative models from leading manufacturers of All-on-4 implant systems. Pricing varies by region, surgical complexity, and prosthetic materials. Average cost per arch ranges from $12,000–$18,000 for Standard systems and $18,000–$25,000 for Advanced systems, inclusive of surgical placement, implants, abutments, and fixed prosthesis.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing All-on-4 Implant Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Introduction: Navigating China’s All-on-4 Implant Market
As global demand for cost-effective All-on-4 solutions grows, China remains a strategic sourcing hub. However, 2026 market dynamics require rigorous due diligence due to increased regulatory scrutiny (EU MDR 2024 updates, FDA QSIT 2025) and supply chain volatility. This guide provides a technical framework for determining verified average costs while mitigating compliance risks. Note: “All-on-4 systems” include implants, abutments, surgical guides, and prosthetic components.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certificate checks are insufficient in 2026. Implement this technical verification protocol:
| Verification Layer | 2026 Compliance Requirement | Actionable Verification Method |
|---|---|---|
| ISO 13485:2023 | Valid certification covering specific implant manufacturing processes (ISO 13485:2016 no longer accepted in EU) | Request certificate + scope of approval document. Cross-check with IAF CertSearch database using certificate number. |
| EU CE Marking | MDR 2024-compliant Technical Documentation (Annex II/III) + EU Authorized Representative | Demand full EU DoC + EUDAMED SRN number. Verify REP details via EUDAMED portal (mandatory since Jan 2025). |
| Implant-Specific | ASTM F136/F1472 titanium compliance + 10-year clinical data | Require mill test reports + FDA 510(k) or CE clinical evaluation report excerpts. Reject “generic” implant claims. |
Shanghai Carejoy Verification Protocol
As a 19-year OEM/ODM specialist, Carejoy provides:
- Real-time access to their digital compliance dashboard (updated quarterly)
- MDR 2024-compliant Technical Files for All-on-4 systems (SRN: CJD-2026-IMPL)
- On-site audit scheduling via their Baoshan District facility (ISO 13485:2023 cert # CN-SH-2026-088)
Step 2: Negotiating MOQ – Strategic Volume Planning
MOQ structures directly impact actual per-unit cost. Avoid these 2026 pitfalls:
| MOQ Factor | Industry Standard (2026) | Cost-Saving Strategy |
|---|---|---|
| Base MOQ | 50 units (full All-on-4 system) | Negotiate phased fulfillment: 25 units initial shipment + 25 units within 90 days (avoids warehousing fees) |
| Customization | +15-25% cost for clinic-branded components | Use Carejoy’s modular OEM library – pre-certified abutments/surgical guides reduce setup fees by 40% |
| Cost Breakdown | Average base cost: $850-$1,200/system (FOB Shanghai) | Target $920-$1,050 by bundling with complementary equipment (e.g., CBCT) |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Shipping terms constitute 18-22% of landed cost. 2026 best practices:
| Term | 2026 Cost Components | When to Use |
|---|---|---|
| FOB Shanghai | • Base product cost • Local China freight to port • Excludes: Ocean freight, insurance, destination duties, customs clearance |
Distributors with established logistics partners in China. Requires in-house customs expertise. |
| DDP (Your Clinic) | • Base product cost • All freight/insurance • Mandatory 2026 EU VAT prepayment • FDA Prior Notice fees • Final-mile delivery |
Clinics without import experience. Eliminates 14-21 day customs delays (critical for implant sterility timelines). |
Shanghai Carejoy: Your 2026 Sourcing Partner
With 19 years of dental export experience, Carejoy mitigates key 2026 sourcing risks:
- Factory-Direct Cost Control: Baoshan District facility eliminates trading company markups (verified average All-on-4 system cost: $985 DDP Frankfurt)
- Regulatory Agility: Dedicated EU MDR/FDA team updates documentation within 72 hours of regulation changes
- MOQ Flexibility: 30-unit MOQ for distributors with 2+ year partnership (vs. industry standard 50)
Connect for Verified 2026 Pricing
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2005
Core Advantage: OEM/ODM All-on-4 Systems with Full Regulatory Traceability
Contact: [email protected] | WhatsApp: +86 15951276160
Request “2026 All-on-4 Cost Validation Kit” (Includes: MDR-compliant spec sheet, DDP calculator, audit checklist)
Disclaimer: Average costs reflect Q1 2026 market conditions. Final pricing subject to titanium alloy spot prices (LME-linked) and destination country regulatory fees. This guide provides technical sourcing methodology; not a price guarantee.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Key Considerations When Evaluating the Average Cost of All-on-4® Dental Implant Systems in 2026
Frequently Asked Questions (FAQs)
As dental clinics and distributors plan procurement strategies for 2026, understanding the total cost of ownership (TCO) for All-on-4® dental implant systems is essential. Below are five critical technical and operational FAQs addressing voltage compatibility, spare parts availability, installation requirements, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing equipment for All-on-4® implant procedures in 2026? | All surgical equipment used in All-on-4® procedures—including dental implant motors, surgical guides, and imaging systems—must comply with local voltage standards (e.g., 110–120V in North America, 220–240V in Europe and Asia). Ensure that motors and digital planning stations are equipped with dual-voltage capability or come with compatible transformers. Verify CE, FDA, or ISO 60601-1 certification for electrical safety to prevent equipment failure and ensure patient safety. |
| 2. Are spare parts for All-on-4® surgical kits and prosthetic components readily available, and how does this affect long-term cost? | Yes, OEM spare parts—including surgical drill guides, healing caps, multi-unit abutments, and prosthetic housings—must be available through authorized distributors. In 2026, clinics should prioritize implant systems with global spare parts logistics networks. Limited availability can lead to extended downtimes and increased indirect costs. Confirm with suppliers minimum guaranteed spare parts availability (ideally 10+ years post-purchase) to ensure long-term serviceability and reduce lifecycle costs. |
| 3. What installation and calibration procedures are required for All-on-4®-compatible digital workflows (e.g., CBCT, CAD/CAM)? | Installation of All-on-4® digital systems requires on-site calibration by certified biomedical engineers or OEM technicians. This includes integration of CBCT scanners, intraoral scanners, and milling units into a unified digital workflow. Proper calibration ensures accurate implant placement and prosthetic fit. Installation typically takes 1–2 days and may incur additional fees; however, bundled service packages are available from leading providers to reduce upfront costs and ensure compliance with ISO 13485 standards. |
| 4. Does the warranty cover both the implant components and the prosthetic restoration in an All-on-4® system? | Warranty terms vary by manufacturer. In 2026, most premium All-on-4® systems offer a 10-year warranty on titanium implants and abutments against mechanical failure or osseointegration loss. Prosthetic restorations (e.g., acrylic or zirconia bridges) typically carry a 2–5 year warranty. Clinics must review warranty exclusions—such as bruxism-related damage or improper installation. Distributors should ensure end-users receive full warranty documentation and register implants with the manufacturer for traceability and service claims. |
| 5. How do voltage fluctuations and power surges impact the longevity of All-on-4® digital planning equipment, and what protective measures are recommended? | Voltage fluctuations can degrade sensitive electronics in CBCT units, surgical navigation systems, and implant motors. To protect equipment, clinics should install medical-grade surge protectors and uninterruptible power supplies (UPS) rated for dental imaging devices. Systems compliant with IEC 60601-1-2 (4th edition) offer enhanced electromagnetic compatibility (EMC) and resilience. Factoring in protective infrastructure is essential when calculating the total cost of ownership for All-on-4® digital ecosystems. |
Need a Quote for Average Cost Of All On 4 Dental Implants?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


