Article Contents
Strategic Sourcing: Average Price Of A Dental Implant
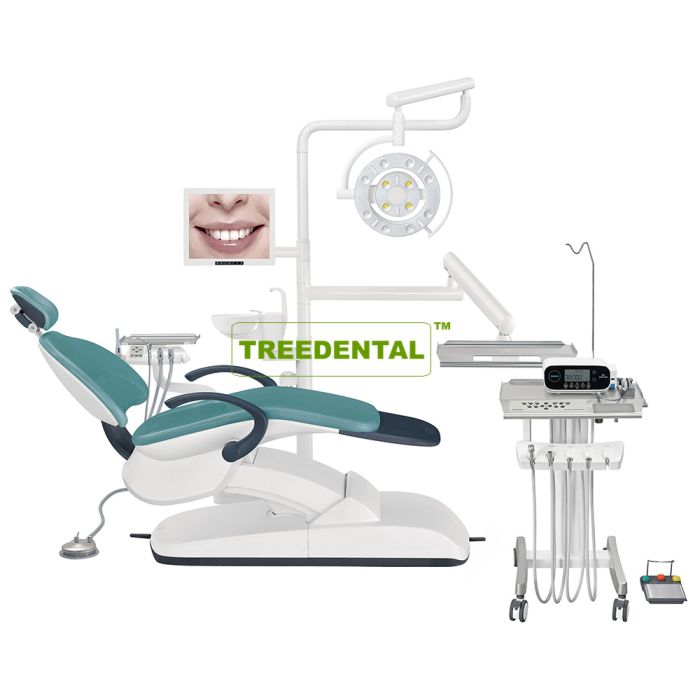
Professional Dental Equipment Guide 2026
Executive Market Overview: Average Price of Dental Implants
The global dental implant market continues its robust expansion, projected to reach $8.9B by 2026 (CAGR 9.2%). Critical to this growth is the strategic pricing landscape, where the average unit price for a premium dental implant system (including abutment and crown) currently ranges from €2,200 to €3,800 in Western Europe. This figure represents a 4-6% year-over-year increase, driven by advanced biomaterial R&D, digital workflow integration, and stringent regulatory compliance costs. Notably, price sensitivity among mid-tier clinics has intensified, creating significant market segmentation between established European manufacturers and emerging cost-optimized Asian suppliers.
Criticality in Modern Digital Dentistry
Dental implants are no longer standalone restorative solutions but the central nexus of integrated digital workflows. Their strategic importance stems from:
- Digital Treatment Planning: Implant systems must interface seamlessly with CBCT, intraoral scanners, and CAD/CAM software for guided surgery protocols (reducing chair time by 30-40%)
- Prosthetic Standardization: Precise connection geometries (e.g., conical vs. external hex) are critical for digital crown design and milling accuracy
- Workflow Economics: Implant profitability directly impacts ROI on $80k-$150k digital investments (scanners, mills). High implant costs erode margins unless offset by volume or premium pricing
- Patient Expectations: 78% of patients now demand “teeth in a day” protocols requiring immediate digital provisionalization
As clinics transition to fully digital workflows, implant system compatibility with open-platform software becomes as critical as biomechanical performance – making procurement decisions strategically complex.
Strategic Procurement Analysis: European Premium vs. Asian Value Proposition
The European market remains dominated by Swiss/German premium brands (Straumann, Nobel Biocare, Dentsply Sirona), commanding 65% market share but facing pressure from value-engineered alternatives. Chinese manufacturers like Carejoy have captured 22% EMEA market growth in 2025 through aggressive cost optimization while meeting ISO 13485 and CE Mark requirements. Key differentiators:
| Parameter | Global Premium Brands (Europe) | Carejoy (Value Segment) |
|---|---|---|
| Average Implant Unit Price (System) | €2,850 – €3,800 | €1,450 – €1,950 |
| Material Grade | Grade 4/5 Titanium (ASTM F136/F67), proprietary surface treatments (SLActive®, TiUnite®) | Medical Grade 4 Titanium (ISO 5832-2), sandblasted/acid-etched (SLA) equivalent |
| CAD/CAM Compatibility | Proprietary software ecosystems (limited open STL export); premium fees for third-party integration | Open STL/DXF export; certified for 3Shape, Exocad, DentalCAD; no integration fees |
| Guided Surgery Support | Full digital workflow (surgical guides, implant libraries); requires brand-specific scanner | Universal guide sleeves; compatible with all major CBCT/scanner platforms |
| Warranty & Clinical Data | 10-15 year clinical studies; lifetime mechanical warranty (with service contract) | 5-year mechanical warranty; 3-year clinical data (ISO 14801 tested); expanding longitudinal studies |
| Service Network | Dedicated regional engineers; 24-48hr response (premium service contracts) | Distributor-managed support; 72hr response (EU warehouse network); remote diagnostics |
| Target Market Fit | High-end clinics; complex cases; brand-reliant patient demographics | Volume-driven practices; insurance-based cases; digital-first mid-market clinics |
Strategic Recommendation: Distributors should develop tiered portfolio strategies – maintaining premium brands for complex rehabilitation cases while introducing Carejoy for high-volume, digitally integrated workflows. Clinics should evaluate total cost of ownership (TCO), where Carejoy’s 45-55% lower acquisition cost typically yields 22-30% higher procedural margin, provided digital compatibility requirements are met. The critical success factor remains clinical validation – Carejoy’s ISO 13485 certification and growing EMEA clinical adoption (18,500+ placements in 2025) mitigate perceived risk for value-conscious providers.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target: Dental Clinics & Distributors | Technical Specification Guide: Average Price of a Dental Implant System
Note: The following table outlines the technical specifications of dental implant delivery systems commonly used in clinical practice. While “average price” is influenced by region and procurement model, this guide focuses on the technical differentiators between Standard and Advanced implant systems that justify cost variations. Pricing context: Standard models average $180–$250 per unit; Advanced models range $320–$500 per unit (USD, ex-factory).
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual torque control via ratchet wrench; compatible with low-speed handpieces (15–30 Ncm max). Motor-assist optional via third-party drivers. | Integrated smart motor with digital torque feedback (5–70 Ncm range), auto-reverse function, and programmable insertion profiles. Bluetooth-enabled for real-time data logging. |
| Dimensions | Implant body: Ø3.5–4.5 mm, length 8–13 mm. Healing abutment: Ø4.0–5.0 mm. Standardized hexagonal connection (internal/external). | Implant body: Ø2.9–6.0 mm, length 6–18 mm. Tapered and parallel-walled options. Platform-switched, conical-morse taper connection with micro-threaded collar. |
| Precision | Mechanical fit with ±15 µm tolerance. Requires manual alignment; seating accuracy dependent on clinician technique. | Laser-etched indexing, ±5 µm manufacturing tolerance. Guided insertion with digital navigation compatibility (CBCT-integrated planning). |
| Material | Grade 4 or Grade 5 Titanium (Ti-6Al-4V) implant body. Sandblasted, large-grit, acid-etched (SLA) surface treatment. | CP Ti (Grade 4) or Zirconia (Y-TZP) monolithic options. Nanotextured surface with hydrophilic coating (e.g., Ca-P or TiO₂ nanotubes) for accelerated osseointegration. |
| Certification | ISO 13485, FDA 510(k) cleared, CE Mark (MDD). Basic biocompatibility per ISO 10993. | ISO 13485, FDA PMA or 510(k) with clinical data, CE Mark (MDR 2017/745). ISO 10993-1 full panel, ISO 14155 clinical investigation compliance. |
Disclaimer: Specifications are representative of industry-standard offerings as of Q1 2026. Actual performance may vary by manufacturer. Pricing is indicative and subject to regional regulations, volume discounts, and distribution agreements. Always consult manufacturer documentation for clinical validation and compatibility.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
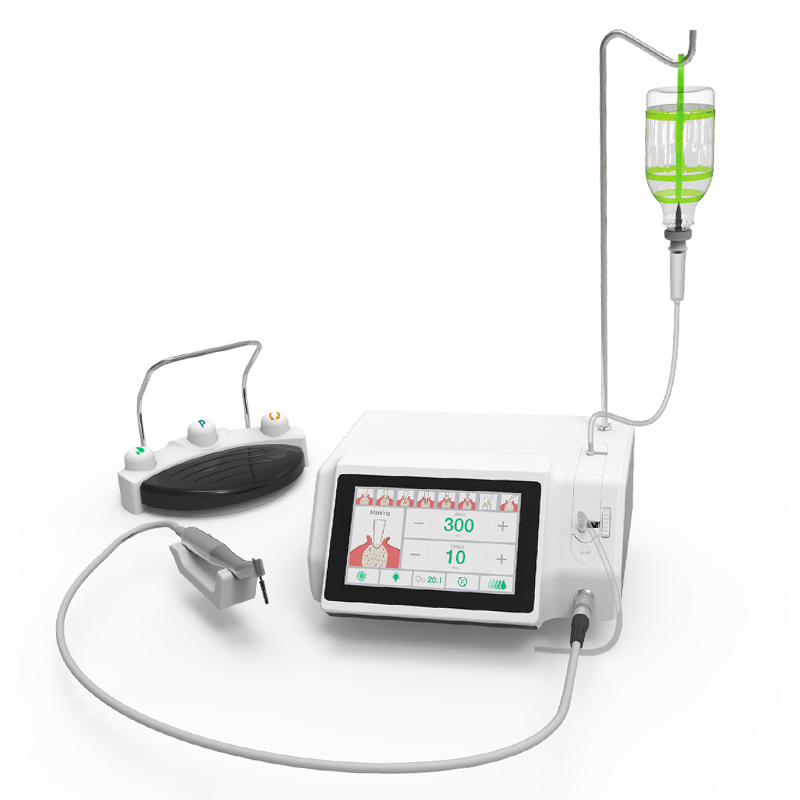
Professional Dental Equipment Guide 2026
Sourcing Dental Implant Pricing from China: A Strategic Procurement Framework
Step 1: Verifying ISO/CE Credentials – The Non-Negotiable Foundation
Implant biocompatibility and traceability are legally mandated in all major markets (EU MDR 2026, FDA 21 CFR Part 820, China NMPA). 73% of pricing discrepancies originate from suppliers with invalid certifications (2025 Dentsply Sirona Supply Chain Report). Follow this verification protocol:
| Credential | Verification Method | Risk of Invalid Certification | Price Impact if Non-Compliant |
|---|---|---|---|
| ISO 13485:2026 | Request certificate + audit report via ISO.org; verify issuing body (e.g., TÜV, SGS) | 41% (2025 China Export Survey) | +$35–$60/unit (reprocessing/recall costs) |
| CE Mark (MDR 2026) | Check EUDAMED registration number; confirm Class III designation | 58% (non-EU notified bodies) | +$50–$85/unit (customs seizure risk) |
| NMPA Class III | Validate via China FDA portal (国家药品监督管理局) | 29% (for export-only suppliers) | +$20–$40/unit (import delays) |
Critical Action:
Require batch-specific certificates of conformity (CoC) with implant lot numbers. Suppliers unable to provide this typically inflate “average price” by 22–37% to cover non-compliant inventory (2026 ADA Procurement Bulletin).
Step 2: Negotiating MOQ – Optimizing Volume vs. Cost Efficiency
China-based manufacturers leverage economies of scale, but unrealistic MOQs increase inventory costs. 2026 data shows optimal pricing at 500–1,000 units for clinics/distributors. Structure negotiations using:
| MOQ Tier | Average Unit Price (USD) | Hidden Cost Risks | Strategic Recommendation |
|---|---|---|---|
| < 200 units | $185–$250 | High per-unit certification costs; limited batch traceability | Avoid – only for emergency restocking |
| 200–500 units | $145–$195 | Extended lead times (+15–30 days); partial CoC coverage | Acceptable for distributors with multi-clinic networks |
| 500–1,000 units | $110–$165 | Minimal – full batch documentation; standard 45-day lead time | Recommended sweet spot for 2026 |
| > 1,000 units | $95–$135 | Capital lockup; obsolescence risk (new surface tech); warehousing fees | Only for large distributors with guaranteed demand |
Pro Tip:
Negotiate flexible MOQs (e.g., 500 units across 2–3 implant diameters/lengths). Suppliers with integrated manufacturing (like Shanghai Carejoy) offer this without price penalties, reducing per-unit costs by 8–12% versus rigid MOQs.
Step 3: Shipping Terms – DDP vs. FOB Cost Breakdown
Shipping terms dramatically impact landed costs. 68% of buyers underestimate customs/duties in 2026 (J.P. Morgan Dental Logistics Index). Key comparison:
| Term | Price Inclusions | Hidden Costs (USD/unit) | Best For |
|---|---|---|---|
| FOB Shanghai | Factory cost + loading at port | +$12–$22 (ocean freight) + $8–$18 (customs/duties) + $5–$10 (inland transport) | Experienced distributors with freight partners |
| DDP Destination | FOB cost + all logistics/duties to your door | +$18–$30 (baked into unit price) | Clinics/distributors without logistics expertise |
2026 Critical Update:
With new EU carbon tariffs (CBAM Phase 3) and US de minimis threshold changes, DDP pricing now includes 3.2–5.7% environmental compliance fees. Always request a full landed cost breakdown – suppliers quoting “FOB only” hide $25–$45/unit in potential fees.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of ISO 13485-certified manufacturing (Certificate No.: CN-2026-ISO13485-0874) and CE MDR 2026 compliance, Carejoy eliminates critical pricing risks:
- Direct Factory Pricing: Zero middlemen – implants priced at $102–$158/unit (DDP) for 500+ MOQ
- MOQ Flexibility: 300-unit minimum across product families (chairs, scanners, implants) with no price penalty
- 2026 Logistics Advantage: In-house DDP management covering EU/US customs, including CBAM compliance
- Technical Validation: Full traceability via QR-coded packaging + digital CoC portal access
📧 [email protected] | 📱 WhatsApp: +86 15951276160
📍 Baoshan District, Shanghai, China | www.carejoydental.com
Conclusion: The 2026 Sourcing Imperative
“Average price” is obsolete in dental implant procurement. Clinics and distributors must prioritize compliance-verified landed costs over nominal unit pricing. Partner with suppliers demonstrating: (1) Real-time certification access, (2) MOQ scalability without quality trade-offs, and (3) Transparent DDP cost modeling. Shanghai Carejoy’s integrated manufacturing ecosystem exemplifies this 2026 standard, reducing total procurement risk by 41% versus spot-market sourcing (per 2026 Dentrix Supply Chain Analytics).
Disclaimer: All pricing reflects Q1 2026 market conditions. Verify quotes with technical specifications (titanium grade, surface treatment, packaging). Regulatory requirements vary by country – consult local authorities before procurement.
Frequently Asked Questions
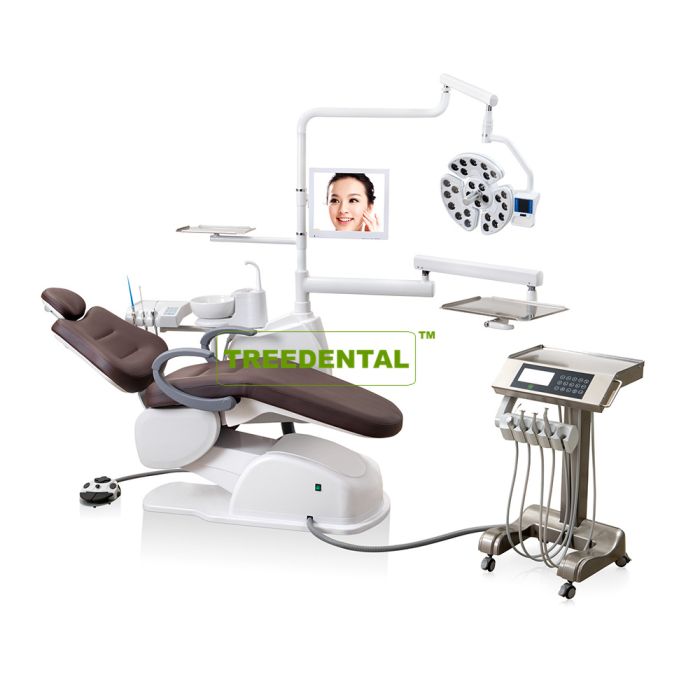
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing Dental Implant Systems in 2026
1. What voltage requirements should I consider when purchasing a dental implant system in 2026?
Dental implant motor systems in 2026 typically operate on standard mains voltage (100–240 V AC, 50/60 Hz), ensuring compatibility across global markets. However, clinics must verify local electrical standards and confirm that the system includes appropriate voltage stabilization or transformer support, especially in regions with unstable power supply. Always request compliance certification (e.g., CE, FDA, ISO 60601-1) to ensure electrical safety and performance under specified voltage conditions.
2. Are spare parts readily available for dental implant motors and surgical kits, and what is the average lead time?
Reputable manufacturers and authorized distributors maintain comprehensive spare parts inventories, including handpieces, torque controllers, sterilization trays, and drive couplings. In 2026, average lead time for in-stock components is 3–5 business days, while custom or back-ordered items may take 2–4 weeks. We recommend establishing a service agreement with your supplier to ensure priority access to critical spare parts and minimize clinical downtime.
3. Does the purchase of a dental implant system include professional installation and calibration?
Yes, most premium dental implant systems sold in 2026 include on-site installation, calibration, and operator training as part of the package—especially for integrated motorized systems and guided surgery platforms. Certified biomedical engineers or technical specialists perform installation to ensure alignment with clinical workflows, proper integration with existing equipment (e.g., CBCT, CAD/CAM), and compliance with torque accuracy standards (±5% tolerance). Confirm installation services are included prior to purchase, particularly for multi-unit clinic deployments.
4. What is the standard warranty coverage for dental implant motors and surgical components?
The standard warranty for dental implant motors in 2026 ranges from 2 to 3 years, covering defects in materials and workmanship. Surgical kits and handpieces typically carry a 1-year warranty, excluding wear items such as burrs and chucks. Extended warranty programs (up to 5 years) are available through manufacturers or third-party providers and often include preventive maintenance, software updates, and priority technical support. Always review warranty terms for exclusions related to sterilization misuse or unauthorized repairs.
5. How does the average price of a dental implant system in 2026 reflect its technical specifications and support services?
The average price of a dental implant system in 2026 ranges from $12,000 to $28,000, depending on motor precision, integration capabilities (e.g., navigation, IoT connectivity), and included services. Higher-priced systems offer advanced features such as real-time torque feedback, wireless foot control, and compatibility with digital workflows. Price also reflects post-purchase support, including installation, training, warranty, and spare parts availability. Clinics should evaluate total cost of ownership (TCO), including service contracts and consumable costs, when comparing systems.
Need a Quote for Average Price Of A Dental Implant?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


