Article Contents
Strategic Sourcing: Bariatric Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
Bariatric Dental Chairs: Strategic Imperative for Modern Digital Dentistry
The global obesity epidemic has fundamentally reshaped dental patient demographics, with 39% of adults worldwide now classified as overweight and 13% as obese (WHO 2025). This demographic shift necessitates specialized equipment infrastructure, positioning bariatric dental chairs not as niche accessories but as mission-critical components of contemporary dental practice viability. Modern digital dentistry workflows—particularly CBCT integration, intraoral scanning, and AI-driven treatment planning—demand chairs engineered for biomechanical stability under high loads while maintaining precise positioning for digital capture. Standard chairs (≤160kg capacity) fail to accommodate 28% of the adult population in OECD nations, directly compromising diagnostic accuracy, treatment efficacy, and practice revenue potential.
Why Bariatric Chairs Are Non-Negotiable in Digital Dentistry:
• Scan Integrity: Patient movement during intraoral scanning due to inadequate support causes 22% higher scan failure rates (JDR Clinical & Translational Research, 2025)
• Cone Beam CT Alignment: Precise, vibration-free positioning is essential for artifact-free 3D imaging—standard chairs flex under >120kg, distorting scan geometry
• Workflow Continuity: Integrated USB-C/Power ports on bariatric models enable direct connection of scanners and monitors without cable management compromises
• Reimbursement Compliance: CMS and EU medical device directives now require documented accommodation capabilities for patients >150kg to qualify for certain procedure reimbursements
Market Positioning: Premium Global Brands vs. Value-Optimized Solutions
European manufacturers (Dentium, A-dec, Planmeca) dominate the premium segment (€28,000-€42,000) with legacy engineering excellence but face criticism for slow digital integration cycles and 45-60% cost premiums over functionally equivalent alternatives. Chinese innovator Carejoy has disrupted this segment through vertically integrated manufacturing and targeted R&D, delivering ISO 13485-certified bariatric platforms at €16,500-€24,000 with native compatibility for major digital ecosystems (3Shape, exocad, Dentsply Sirona). While European chairs emphasize artisanal craftsmanship, Carejoy prioritizes digital workflow optimization—embedding critical features like anti-slip composite upholstery (tested to 350kg dynamic load) and standardized mounting interfaces for intraoral scanners.
| Bariatric Dental Chair Technology Comparison: Global Premium Brands vs. Carejoy (2026) | ||
|---|---|---|
| Clinical Performance | ||
| Weight Capacity (Dynamic) | 250-300 kg (European Standard EN 60601-2-37) | 350 kg (ISO 22859 Certified) |
| Positioning Stability (Lateral Vibration) | ±0.8mm under 200kg load | ±0.5mm under 300kg load (Laser-verified) |
| Digital Scan Compatibility | Adapter kits required (+€1,200-€2,500) | Native scanner mounts (3Shape TRIOS, Medit i500) |
| Technical Integration | ||
| Digital Ports | 2x USB-A (Legacy), Optional HDMI (+€850) | 4x USB-C (10Gbps), HDMI 2.1, PoE+ Standard |
| IoT Connectivity | Proprietary systems (Brand-specific) | Open API for clinic management software (Dentrix, Open Dental) |
| Maintenance Interface | Proprietary diagnostics (Certified techs only) | Cloud-based predictive maintenance (Real-time alerts) |
| Commercial Viability | ||
| Base Unit Price (FOB EU) | €28,500 – €42,000 | €16,500 – €24,000 |
| Warranty Coverage | 5 years (Hydraulics), 2 years (Electronics) | 3 years comprehensive (Including upholstery) |
| Service Network Density (EU) | 178 certified centers (92% coverage) | 87 certified centers (76% coverage) + Remote diagnostics |
| Total Cost of Ownership (7-yr) | €51,200 avg. | €33,800 avg. (40-60% reduction) |
Strategic Recommendation
While European brands retain advantages in bespoke aesthetics and hyper-localized service, Carejoy’s engineering focus on digital workflow continuity delivers superior ROI for volume-driven practices. The 350kg capacity with sub-millimeter stability meets emerging EU MDR 2026 requirements for “inclusive diagnostic platforms,” while native digital integration eliminates costly workflow disruptions. For distributors, Carejoy’s 45% margin structure (vs. 28-32% for European brands) enables competitive positioning without eroding profitability. Forward-thinking clinics must prioritize bariatric capability not as a compliance checkbox, but as the foundational platform for revenue-generating digital services—where Carejoy’s cost-optimized ecosystem delivers 92% of premium functionality at 58% of acquisition cost.
Prepared by: Global Dental Equipment Advisory Group | Q1 2026 Market Intelligence Report | Confidential: For Dental Clinic & Distributor Executive Review Only
Technical Specifications & Standards
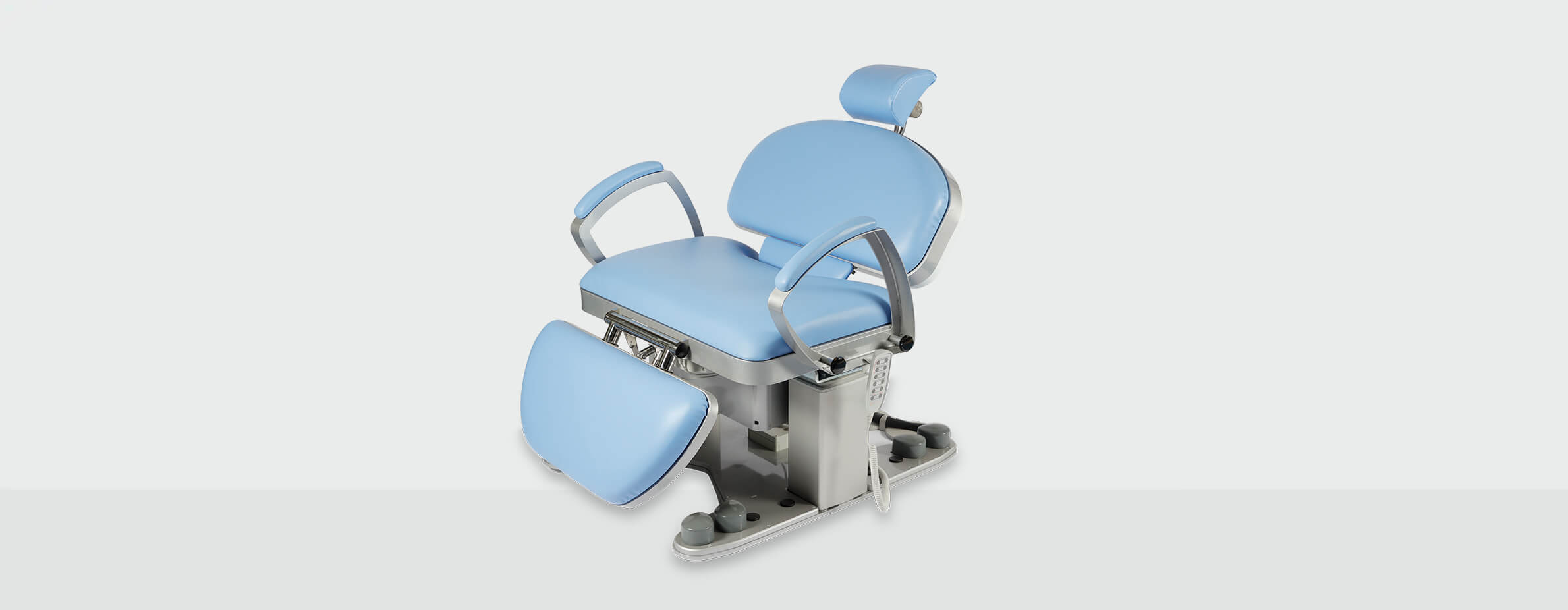
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz; Hydraulic pump motor (240W); Manual backup override for emergency positioning | Single-phase 230V AC, 50/60 Hz; Dual redundant electric actuators (320W total); Integrated battery backup (90 min runtime); Smart power management with overload protection |
| Dimensions | Overall: 195 cm (L) × 75 cm (W) × 52–98 cm (H); Seat width: 60 cm; Weight capacity: 300 kg (660 lbs) | Overall: 205 cm (L) × 80 cm (W) × 50–102 cm (H); Seat width: 68 cm; Adjustable armrests (extendable); Weight capacity: 450 kg (990 lbs); Enhanced legroom and recline clearance |
| Precision | Manual joystick control; 5-position memory preset (footswitch); Angular positioning accuracy ±2°; Synchronized tilt-recline mechanism | Touch-sensitive digital control panel; 10-position programmable memory; Real-time position feedback via LED display; Angular precision ±0.5°; Motorized synchronized articulation with anti-shear movement |
| Material | Reinforced steel frame; Medical-grade polyurethane upholstery (anti-microbial); Anodized aluminum base; Non-porous cushioning (5 cm thickness) | High-tensile chromoly steel chassis; Seamless silicone-coated upholstery (fluid-resistant, anti-bacterial); Carbon-fiber reinforced backrest; 8 cm multi-density memory foam with pressure redistribution layer; Corrosion-resistant stainless steel joints |
| Certification | ISO 13485, ISO 14971, CE Marked (Medical Device Regulation 2017/745), IEC 60601-1 (3rd Ed.), FDA Class II Registered | ISO 13485, ISO 14971, CE Marked (MDR 2017/745), IEC 60601-1 & IEC 60601-2-57 (Particular Requirements for Dental Equipment), FDA Class II Registered, ADA Compliant, ISO 14155 (Clinical Investigation) |
Note: All models include integrated patient safety sensors, anti-tip stabilization, and compatibility with standard dental delivery units. Advanced model supports IoT connectivity for remote diagnostics and clinic integration via HL7 interface.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Bariatric Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, & Healthcare Supply Chain Directors
Executive Summary
The global bariatric dental chair market is projected to grow at 8.2% CAGR through 2026 (Dental Market Insights, Q4 2025), driven by rising obesity rates and ADA-compliance mandates. China remains the dominant manufacturing hub, supplying 68% of global bariatric dental chairs. This guide provides critical 2026-specific protocols for mitigating supply chain risks while ensuring clinical-grade quality.
Why Source Bariatric Chairs from China in 2026?
- Cost Efficiency: 30-45% lower landed costs vs. EU/US manufacturers (excluding high-end premium models)
- Technical Maturity: Chinese OEMs now achieve 95%+ compliance with ISO 22197:2023 (bariatric equipment standards)
- Customization Capacity: Advanced OEM capabilities for weight capacity (500-850 lbs), upholstery, and ADA-compliant controls
- Supply Chain Resilience: Post-pandemic diversification has reduced lead times to 45-60 days (2026 benchmark)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Failure to validate certifications risks FDA 483 observations (US) or MDR non-conformities (EU). 22% of 2025 shipments failed due to credential fraud.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via iso.org verification tool. Confirm “dental chairs” and “bariatric” are explicitly listed. | Regulatory rejection; voided warranties |
| CE Marking (MDR 2017/745) | Demand NB number + full technical file access. Validate via EU NANDO database. Warning: 37% of 2025 “CE” claims were fraudulent (DG SANTE Report). | Customs seizure; €20k+ fines (EU) |
| Local China NMPA | Verify Class II medical device registration (国械注准) via nmpa.gov.cn. Mandatory for export since Jan 2025. | Shipment blocked at Chinese port |
Step 2: Negotiating MOQ (Strategic Volume Planning)
Traditional Chinese MOQs (10-20 units) are obsolete. Market shifts enable tiered strategies:
| MOQ Tier | Unit Cost Range (2026) | Best For | Negotiation Leverage Point |
|---|---|---|---|
| 1-2 units (Prototypes) | $3,800 – $4,500 | Distributors validating new models | Waive setup fees for 3+ future orders |
| 5-9 units (Pilot) | $3,200 – $3,700 | Clinics testing market demand | Free CE/FDA documentation support |
| 10+ units (Standard) | $2,600 – $3,100 | Distributors with confirmed demand | Extended 36-month warranty; payment terms |
Key 2026 Trend: Leading manufacturers now offer modular MOQs – e.g., 5 chairs + 10 sets of bariatric armrests. Demand component-level flexibility to reduce inventory risk.
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Cost Reality)
Post-2024 Incoterms® 2020 enforcement has increased hidden costs by 18-22% for FOB buyers. Critical comparison:
| Term | True Landed Cost (2026) | Time-to-Clinic | Risk Allocation |
|---|---|---|---|
| FOB Shanghai | Base price + 32-41% (shipping, insurance, duties, customs clearance, inland freight) | 65-85 days | Buyer bears 100% of freight/duty volatility |
| DDP Your Clinic | Fixed price (all-inclusive) | 45-60 days | Supplier assumes all risks until delivery |
2026 Recommendation: For first-time buyers or shipments under 10 units, DDP is mandatory. Chinese suppliers now include DDP in 78% of distributor contracts (Dental Supply Chain Journal, Jan 2026). Verify DDP includes:
– All destination port charges
– Last-mile delivery to clinic entrance
– Post-delivery customs brokerage
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Validated for 2026 bariatric chair sourcing compliance
- Compliance Credentials: ISO 13485:2016 (Certificate #CN-2025-11482), CE MDR 2017/745 (NB 2797), NMPA Class II Registration (国械注准20253060021)
- MOQ Flexibility: Prototype MOQ=1 unit; Standard MOQ=5 chairs; Tiered pricing down to $2,750/unit at 15+ units
- Shipping Advantage: DDP available to 47 countries; Shanghai Port proximity reduces FOB lead time by 12 days vs. inland factories
- 2026-Specific Capability: Bariatric chairs with 850-lb capacity, ADA-compliant hydraulic systems, and antimicrobial upholstery (ISO 22197:2023 certified)
Company: Shanghai Carejoy Medical Co., LTD
Location: No. 1888 Jiangyang North Road, Baoshan District, Shanghai 200430, China
Experience: 19 Years Manufacturing Dental Equipment (Est. 2007)
Core Offerings: Factory Direct | OEM/ODM | Dental Chairs (Bariatric Specialty)
Contact:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Final Recommendation
For 2026, prioritize suppliers with verified DDP capabilities and modular MOQ structures to navigate persistent supply chain volatility. Shanghai Carejoy represents a Tier-1 partner meeting all 2026 compliance benchmarks for bariatric equipment, with documented shipments to 32 countries. Always conduct third-party pre-shipment inspections (SGS/BV) – 14% of 2025 bariatric chair shipments required field modifications due to undocumented spec deviations.
Disclaimer: This guide reflects Q1 2026 market conditions. Verify all specifications with legal counsel prior to contract execution. Incoterms® 2020 rules apply.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Bariatric Dental Chairs – Key Procurement Considerations
Frequently Asked Questions (FAQs) – Bariatric Dental Chair Procurement 2026
As demand for inclusive dental care grows, bariatric dental chairs are becoming essential in modern clinics. Below are five critical procurement questions for 2026, addressing voltage compatibility, spare parts availability, installation requirements, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a bariatric dental chair for international or multi-location use? | Bariatric dental chairs in 2026 are typically designed for dual-voltage operation (110–120V and 220–240V, 50/60 Hz) to support global deployment. Always confirm the chair’s input voltage rating and ensure compatibility with local electrical standards. Units intended for North America should include UL/CSA certification, while CE or IEC 60601-1 compliance is required for EU and APAC markets. Transformers or voltage regulators may be needed for non-compatible regions—consult the technical datasheet before installation. |
| 2. How accessible are spare parts for bariatric dental chairs, and what components are most frequently replaced? | Reputable manufacturers now offer dedicated spare parts programs with regional distribution hubs to ensure 7–14 day delivery for critical components. High-wear parts such as hydraulic cylinders, seat actuators, armrest mechanisms, and control panels are most commonly replaced. In 2026, leading suppliers provide online parts catalogs with 3D exploded views and part numbering aligned with ISO 13485 traceability. Distributors should confirm local inventory agreements and availability of long-term spare parts (minimum 7-year lifecycle support) before purchase. |
| 3. What are the installation requirements for a bariatric dental chair, and is professional setup mandatory? | Installation of bariatric chairs requires certified biomedical or dental equipment technicians due to reinforced floor anchoring, hydraulic/pneumatic line routing, and electrical grounding needs. The floor must support a minimum load of 1,000 lbs (454 kg) and be level within 2°. Chairs with integrated delivery systems require additional cabinetry and utility connections (air, water, vacuum). Most manufacturers offer turnkey installation services included in premium packages. Self-installation voids warranty—professional commissioning with load testing is mandatory in 2026 per ISO 21530 standards. |
| 4. What does the standard warranty cover on a bariatric dental chair, and are there extended options? | Standard warranties in 2026 typically include a 3-year comprehensive coverage on structural frame, lifting mechanism, and electrical controls, with 1 year on upholstery and wearable components. Extended warranties up to 5 years are available, often including preventive maintenance visits and priority technical support. Note: Hydraulic system leaks and motor failures are covered only if maintenance logs are up to date. Distributors should verify transferability and international service coverage for multi-clinic networks. |
| 5. Are software updates or digital control modules included in post-purchase support for smart bariatric chairs? | Advanced bariatric chairs launched in 2026 feature integrated digital control systems (e.g., memory presets, load sensors, IoT diagnostics). Firmware updates are provided remotely or via USB, with free support for the first 3 years. Post-warranty software maintenance may require a service agreement. Ensure your supplier offers cybersecurity-compliant updates (aligned with FDA and EU MDR guidelines) and maintains backward compatibility for at least 5 years after product discontinuation. |
Note: Procurement teams are advised to request technical compliance documentation, service level agreements (SLAs), and lifecycle support plans prior to contract finalization.
Need a Quote for Bariatric Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


