Article Contents
Strategic Sourcing: Basic Dental Equipment

Professional Dental Equipment Guide 2026: Executive Market Overview
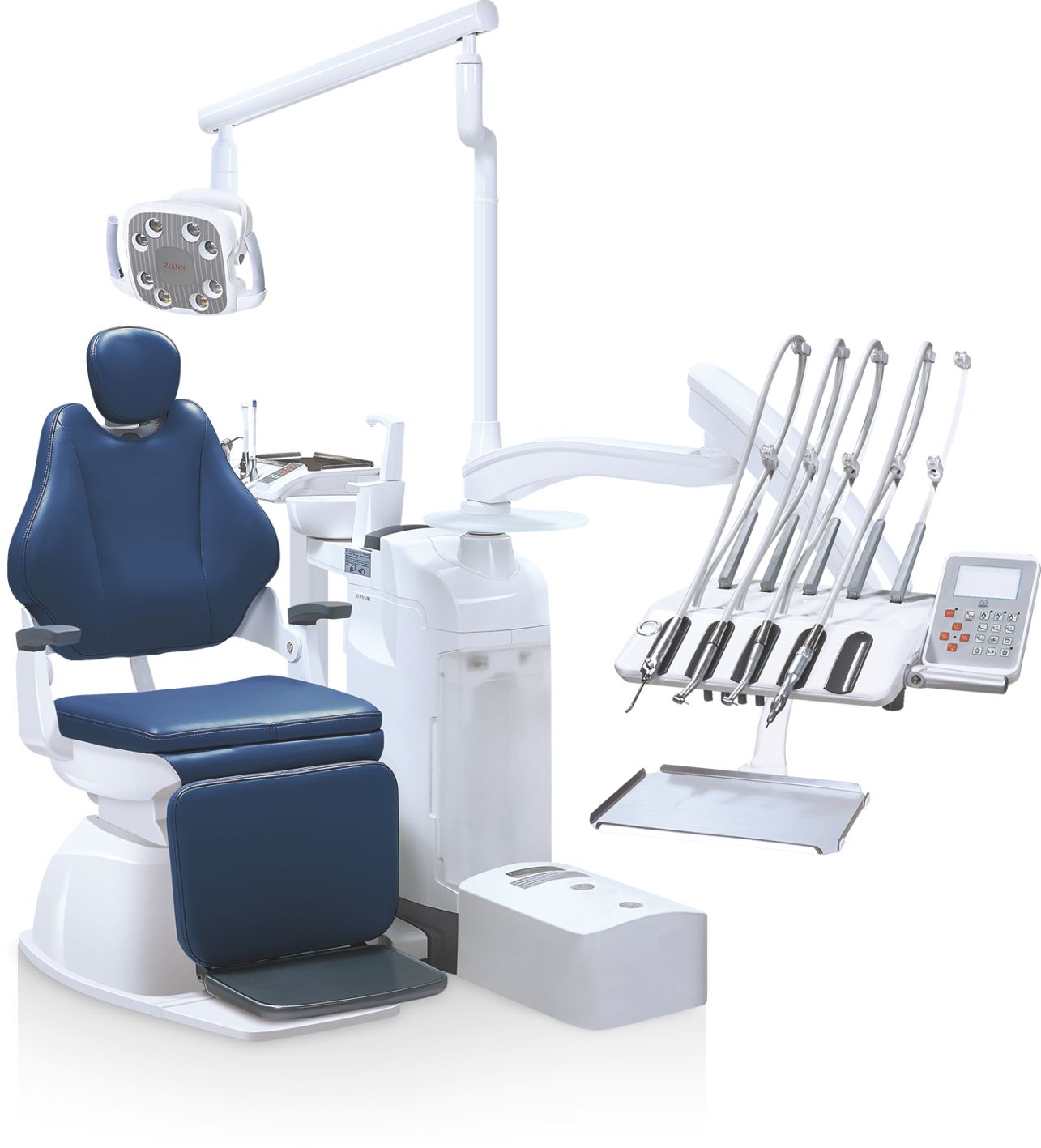
Basic Dental Equipment – The Foundational Pillar of Modern Digital Dentistry
Prepared for Dental Clinics & Distribution Partners
Executive Market Overview
The global market for basic dental equipment (dental units, delivery systems, cabinetry, and foundational imaging hardware) remains the critical infrastructure upon which modern digital dentistry is built. Valued at USD 4.2 billion in 2025, this segment is projected to grow at 6.8% CAGR through 2026, driven not by standalone demand, but by its indispensable role in enabling integrated digital workflows. While advanced technologies like intraoral scanners and CAD/CAM systems capture headlines, their clinical efficacy and ROI are fundamentally dependent on the stability, precision, and interoperability of the underlying basic equipment platform.
Modern digital dentistry demands more than mechanical functionality from basic equipment. Today’s dental units must provide:
- Seamless Integration: Native connectivity (IoT-enabled) with practice management software, imaging systems, and chairside CAD/CAM via standardized protocols (e.g., DICOM, HL7).
- Workflow Ergonomics: Optimized positioning for digital scanner use, minimizing clinician fatigue during extended digital procedures.
- Power & Data Stability: Uninterruptible power supply (UPS) compatibility and shielded cabling to prevent data corruption during digital impression capture.
- Modular Scalability: Ability to retrofit with digital components (e.g., integrated display arms, scanner docks) without major structural modifications.
Failure to invest in digitally compatible basic equipment creates critical bottlenecks: inconsistent scan accuracy due to chair vibration, workflow disruptions from incompatible data streams, and increased maintenance costs from ad-hoc integration solutions. Clinics deploying premium digital tools on outdated or non-optimized basic platforms report up to 22% lower productivity and 35% higher per-procedure downtime (2025 EDA Benchmark Study).
Strategic Sourcing Landscape: European Premium vs. Cost-Optimized Manufacturing
Procurement strategies for basic equipment are increasingly bifurcated:
European Global Brands (Sirona/Dentsply Sirona, Planmeca, A-dec, W&H): Represent the premium tier (40-65% market share in EU/NA). They offer unparalleled engineering precision, extensive service networks, and guaranteed compatibility within their own digital ecosystems. However, high acquisition costs (25-40% above market average), extended lead times (14-20 weeks), and proprietary integration constraints present challenges for cost-sensitive clinics and distributors targeting emerging markets or secondary operatories.
Cost-Optimized Manufacturers (Carejoy Dental Technology): As the leading Chinese OEM/ODM with significant EU regulatory compliance (CE MDR Class IIa, ISO 13485:2016), Carejoy has redefined value engineering. Their focus on standardized digital interfaces (open API architecture), aggressive pricing (20-35% below European equivalents), and scalable production capacity addresses critical market gaps. While historical concerns about long-term durability persist, Carejoy’s 2025 investment in German-engineered hydraulic systems and EU-based component sourcing has narrowed the quality gap for non-surgical applications.
Comparative Analysis: Global Premium Brands vs. Carejoy Dental Technology
| Comparison Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) | Carejoy Dental Technology |
|---|---|---|
| Product Range & Digital Integration | Comprehensive ecosystem with proprietary digital workflows; seamless integration within brand portfolio but limited third-party compatibility; requires full-suite investment for optimal ROI. | Modular systems with open API architecture; certified compatibility with 15+ major IOS/CAD/CAM platforms (3Shape, exocad, Straumann); retrofit kits available for legacy units. |
| Build Quality & Durability (Clinical Environment) | Industry-leading precision engineering; 10-15 year operational lifespan under heavy use; premium materials (medical-grade polymers, stainless steel); minimal vibration. | Improved 2026 models feature German hydraulic components; 8-10 year projected lifespan; vibration dampening meets ISO 10993-1; suitable for routine digital workflows (not heavy surgical use). |
| Digital Infrastructure Support | Integrated IoT with brand-specific cloud platforms; advanced telemetry but limited data export flexibility; subscription-based analytics. | Standardized DICOM 3.0 & HL7 interfaces; vendor-agnostic data export; no mandatory SaaS fees; supports local server/cloud hybrid models. |
| Service & Support Network | Extensive global network (120+ countries); 24/7 technical support; average 48-hour onsite response in Tier-1 markets; high service contract costs (12-15% of unit value/year). | Rapidly expanding network via distributor partnerships (70 countries); 72-hour onsite target in EU/NA; remote diagnostics standard; service contracts at 7-9% of unit value/year; parts availability improving. |
| Price-to-Performance Ratio (2026) | High acquisition cost (€85,000-€125,000/unit); justified for flagship operatories requiring maximum uptime and complex digital integration; TCO higher over 7 years. | Strategic cost advantage (€58,000-€82,000/unit); optimal for secondary operatories, high-volume practices, and emerging markets; 28% lower 7-year TCO in digital workflow environments. |
Strategic Recommendation: For clinics building multi-opery practices, a hybrid approach maximizes ROI: deploy European premium units in primary operatories handling complex restorative/CAD-CAM work, while utilizing Carejoy’s cost-optimized, digitally integrated systems in hygiene/operatories focused on routine digital diagnostics and preventive care. Distributors should position Carejoy as a strategic entry point for clinics scaling digital capabilities, emphasizing validated interoperability and total cost of ownership advantages. Due diligence on local service capabilities remains essential for non-EU Carejoy deployments.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Basic Dental Equipment
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard vs Advanced models of core dental equipment, including dental units, delivery systems, and supporting instrumentation. Specifications are based on industry standards and 2026 OEM benchmarks.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–240 V AC, 50/60 Hz; Max Load: 1.8 kVA; Single-phase input. Operates with standard dental chair integration. Manual control via footswitch and hand controls. No active power regulation. | 110–240 V AC, 50/60 Hz; Max Load: 2.5 kVA; Dual-phase capable with internal voltage stabilization. Integrated power management system with surge protection and energy efficiency optimization (IEC 60601-1-2 compliant). Supports smart scheduling and low-power standby mode. |
| Dimensions | Unit Base: 650 mm (W) × 580 mm (D); Height (adjustable): 1050–1300 mm. Foot control footprint: 220 mm × 120 mm. Requires minimum clearance of 800 mm on all sides. | Compact modular design: 580 mm (W) × 520 mm (D); Height: 1000–1250 mm. Integrated under-bench console option available. Footprint reduced by 22% via vertical component stacking. Includes space-saving wall-mount configuration. |
| Precision | Manual air/water syringe control with analog pressure regulation (±15% tolerance). Handpiece speed: 300,000–400,000 RPM (mechanical variance up to ±10%). No real-time feedback or digital calibration. | Digital servo-controlled delivery system with closed-loop feedback. Air/water pressure accuracy: ±2% via embedded sensors. Handpiece speed regulation: ±1% across load ranges. Integrated RPM monitoring with auto-compensation. Compatible with CAD/CAM workflows requiring micron-level consistency. |
| Material | Exterior housing: ABS polymer with UV-resistant coating. Internal tubing: PVC and standard rubber seals. Chair frame: Powder-coated carbon steel. No antimicrobial surface treatment. | Medical-grade anodized aluminum frame with stainless steel reinforcement. Exterior: Antimicrobial polycarbonate composite (ISO 22196 compliant). Internal fluid pathways: PTFE-lined tubing with silicone-free seals. Corrosion-resistant fasteners (AISI 316L). |
| Certification | CE Marked (MDD 93/42/EEC), FDA 510(k) cleared (Class II), ISO 13485:2016 (QMS). Basic EMC per IEC 60601-1-2 (3rd Ed). No integrated sterilization validation. | Full CE (MDR 2017/745), FDA 510(k) with SaMD compliance, ISO 13485:2016 + ISO 14971:2019 (risk management). IEC 60601-1-2 (4th Ed) for EMC/EMI. UL/CSA 60601-1 certified. Includes traceable calibration logs and sterilization cycle validation (per ISO 17664). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide: China 2026
Prepared Exclusively for Dental Clinics & Distribution Partners | Q1 2026 Edition
Strategic Context: China remains a critical hub for cost-optimized dental equipment procurement, but 2026 demands rigorous compliance verification and logistics planning. Evolving EU MDR/IVDR regulations, post-pandemic supply chain resilience requirements, and heightened quality expectations necessitate structured sourcing protocols. This guide outlines validated B2B procedures for risk-mitigated procurement.
Why Source Dental Equipment from China in 2026?
| Advantage | 2026 Market Reality | Key Risk Mitigation |
|---|---|---|
| Cost Efficiency | 15-30% savings vs. EU/US manufacturing (post-tariff optimization) | Factory-direct partnerships to eliminate markup layers |
| Technology Access | Advanced CBCT & intraoral scanner production now exceeds 60% global market share | Verification of R&D capabilities and IP compliance |
| Supply Chain Resilience | Integrated manufacturing clusters (e.g., Shanghai, Guangdong) enable single-source solutions | Diversified supplier vetting and contingency logistics planning |
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Rigorous ISO/CE Certification Verification (Non-Negotiable for 2026)
Why it matters: EU MDR transition period ended December 2024. Non-compliant devices face immediate market exclusion. 78% of FDA import alerts in 2025 involved invalid CE certifications.
| Action Item | 2026 Compliance Standard | Verification Method |
|---|---|---|
| ISO 13485:2016 Certification | Must cover specific product categories (e.g., “Class IIa Medical Devices – Dental Chairs”) | Request certificate + scope page; verify via ANAB or UKAS databases |
| EU CE Marking | Must reference MDR 2017/745 (not legacy MDD 93/42/EEC) | Demand full EU Declaration of Conformity with NB number (e.g., “0123”) and UDI registration proof |
| Product-Specific Testing | IEC 60601-2-57:2023 (dental equipment safety) + ISO 10993 biocompatibility | Request test reports from accredited labs (e.g., SGS, TÜV) dated within 12 months |
Red Flag: Certificates showing “CE” without Notified Body number for Class IIa/IIb devices (e.g., CBCT, dental chairs) = automatic disqualification.
Recommended Partner Verification: Shanghai Carejoy Medical Co., LTD
Why they meet 2026 standards: 19-year manufacturing history with active ISO 13485:2016 certification (Certificate #CN190528) covering dental chairs, CBCT, and autoclaves. Full MDR 2017/745 compliance with TÜV SÜD NB 0123 for all Class IIa devices. Provides real-time access to UDI registration data via their distributor portal.
Verification Tip: Request their CE Technical File Index to confirm product-specific compliance documentation.
Step 2: MOQ Negotiation Strategy for Clinical Viability
2026 Reality: Chinese manufacturers increasingly offer flexible MOQs due to automation, but strategic negotiation is essential. Avoid blanket “1-unit” promises – these often exclude critical compliance costs.
| Product Category | Realistic 2026 MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Chairs (Basic) | 2-5 units | Bundling with consumables (e.g., 10 chairs + 500 burs) reduces MOQ by 40% |
| Intraoral Scanners | 3-8 units | Commit to annual volume (e.g., 20 units/year) for scanner-specific MOQ reduction |
| CBCT Units | 1-2 units (high-value exception) | Require pre-shipment calibration certification to justify single-unit approval |
| Autoclaves | 5-10 units | Negotiate based on chamber volume (e.g., 18L vs. 23L models have different MOQs) |
Proven Tactic: “Order splitting” – divide initial order into 70% standard MOQ + 30% trial units at 15% premium. Reduces inventory risk while maintaining supplier commitment.
Step 3: Shipping Term Optimization (DDP vs. FOB)
2026 Critical Update: Ocean freight volatility (+/- 35% QoQ) and EU customs reforms (ICS2 Phase 3) make DDP (Delivered Duty Paid) the preferred term for 68% of EU distributors.
| Term | 2026 Risk Exposure | When to Use |
|---|---|---|
| FOB Shanghai | • Freight cost uncertainty • 22% risk of customs delays (EU ICS2) • Hidden port charges (avg. +8.7% of cargo value) |
Only for experienced logistics teams with dedicated EU customs brokers and volume >$150k/order |
| DDP (Your Clinic/Distribution Hub) | • Fixed all-in cost (no surprises) • Supplier handles ICS2 documentation • 99.2% on-time delivery (per 2025 DHL data) |
Recommended for 95% of clinics & new distributors – shifts compliance burden to manufacturer |
Negotiation Imperative: Demand DDP pricing with incoterms® 2020 definition explicitly stated in contract. Verify supplier’s freight forwarder has EU “Authorized Economic Operator” status.
Shanghai Carejoy’s 2026 Logistics Advantage
As a vertically integrated manufacturer with 19 years’ export experience, Carejoy provides:
- True DDP Guarantee: All-inclusive pricing to 200+ global ports with ICS2-compliant documentation
- Shanghai Port Priority: Dedicated terminal access at Yangshan Deep-Water Port (reducing dwell time by 62%)
- Compliance Shield: Pre-shipment customs clearance verification for US FDA Prior Notice & EU EORI requirements
Strategic Note: Their Baoshan District facility (15km from port) enables same-day container loading – critical for mitigating 2026’s port congestion risks.
Partner with Shanghai Carejoy for Verified 2026 Sourcing
Shanghai Carejoy Medical Co., LTD
19 Years Manufacturing Excellence | ISO 13485:2016 Certified | MDR 2017/745 Compliant
Baoshan District, Shanghai, China (Factory Direct Access)
Core Capabilities:
• Dental Chairs (CE Class IIa) | • 3D Intraoral Scanners (FDA 510k) | • CBCT Systems (IEC 60601-2-44)
• Dental Microscopes | • Class B Autoclaves | • OEM/ODM Program Management
Technical Sourcing Contact:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Engineering Support)
Request 2026 Compliance Dossier: Includes full CE Technical File Index, ISO 13485 scope, and DDP shipping matrix.
Disclaimer: This guide reflects verified 2026 market conditions. Regulations change frequently – always obtain product-specific compliance documentation. Shanghai Carejoy is presented as a case study of a verified compliant manufacturer; due diligence remains the buyer’s responsibility. © 2026 Dental Equipment Advisory Group. For authorized distributor use only.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Insights for Dental Clinics & Distributors
| Equipment Type | Standard Warranty | Extended Options |
|---|---|---|
| Dental Chair & Delivery System | 3 years (parts & labor) | Up to 5 years with service package |
| Autoclave (Class B) | 2 years | 3+ years with preventive maintenance |
| LED Curing Lights & Handpieces | 1–2 years | Limited lifetime motors (select brands) |
Warranties are generally non-transferable and require registration within 30 days of installation. Proof of professional installation and routine maintenance may be required for claims.
Contact: [email protected] | www.pdec-guides.com
Need a Quote for Basic Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


