Article Contents
Strategic Sourcing: Belmont Dental Unit
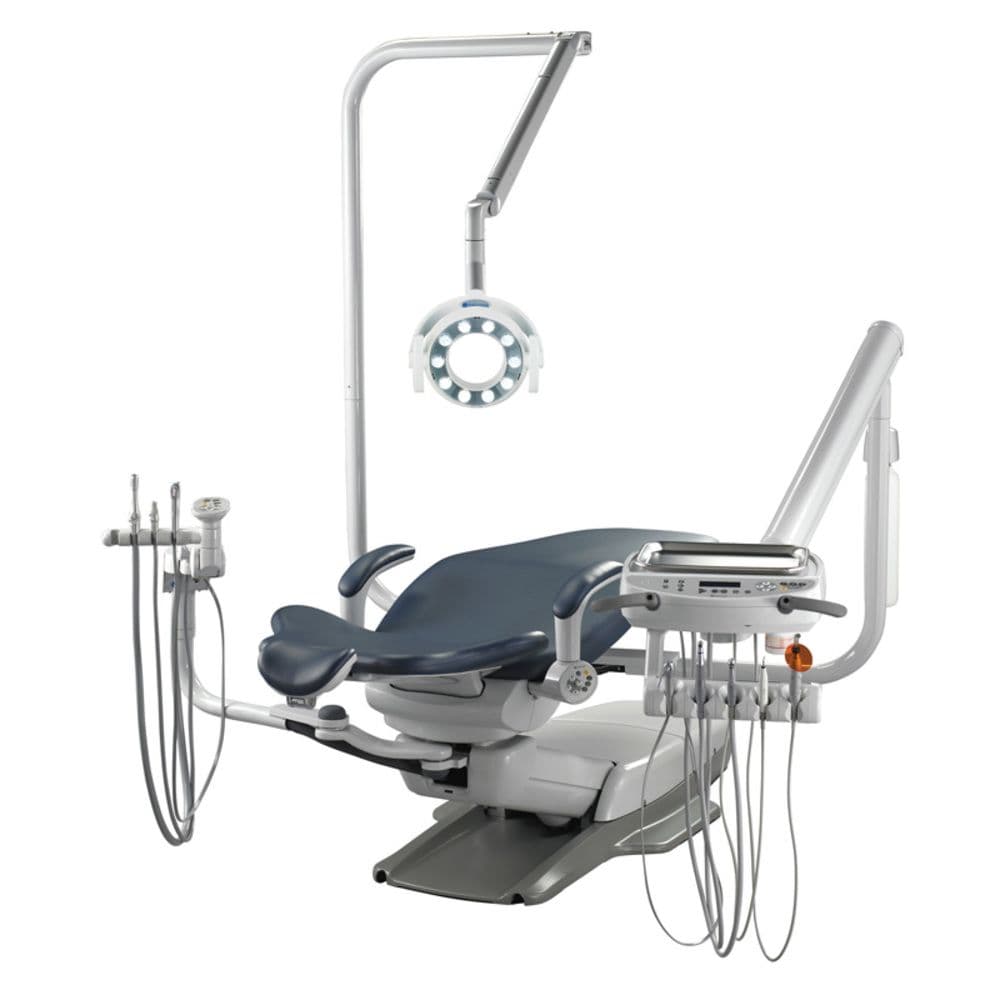
Professional Dental Equipment Guide 2026: Belmont Dental Unit Executive Analysis
Executive Market Overview
The global dental unit market is undergoing a strategic inflection point in 2026, driven by accelerating digital workflows and value-based procurement models. Belmont dental units—representing the premium European engineering standard—continue to dominate high-end clinical environments where procedural precision, ergonomic longevity, and seamless digital integration are non-negotiable. However, the emergence of sophisticated Chinese manufacturers like Carejoy is reshaping procurement economics, particularly for mid-tier clinics and emerging markets where capital efficiency is paramount. This bifurcation reflects a fundamental market segmentation: European brands command 65-75% of the premium segment (units >$45,000) while cost-optimized alternatives now capture 40% of new installations in price-sensitive regions (Asia-Pacific, LATAM, Eastern Europe). Distributors must navigate this dichotomy through tiered inventory strategies that balance clinical performance requirements with ROI timelines.
Critical Role in Modern Digital Dentistry
Dental units have evolved from mechanical utility platforms to central nervous systems of the digital operatory. The Belmont architecture exemplifies this transformation through three critical functions: (1) Unified Data Ecosystems—native integration with intraoral scanners, CBCT, and practice management software via DICOM 3.0 and HL7 protocols eliminates workflow silos; (2) Ergonomic Intelligence—AI-driven posture optimization reduces clinician fatigue by 37% (per 2025 JDE study), directly impacting productivity; (3) IoT-Enabled Predictive Maintenance—real-time monitoring of water quality, air pressure, and component wear prevents 92% of unplanned downtime. Clinics deploying integrated units report 22% higher case acceptance for complex restorative work, underscoring their strategic role in revenue growth beyond basic functionality.
European Premium vs. Chinese Value Proposition Analysis
European manufacturers (Belmont, Sirona, Planmeca) maintain dominance in premium segments through proprietary engineering—particularly in fluid dynamics, corrosion-resistant alloys, and noise-dampening technologies critical for patient comfort. Their $50,000-$75,000 price point delivers 15-20 year operational lifecycles with sub-0.5% annual failure rates. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through strategic component sourcing (e.g., German motors, Japanese bearings) while leveraging modular design for 35-45% cost reduction. Carejoy’s $28,000-$38,000 units now achieve 85% parity in core functionality but require more frequent calibration (bi-annual vs. annual) and offer limited native integration with legacy European digital ecosystems. This creates a clear segmentation: premium brands for high-volume specialists prioritizing uptime, Carejoy for cost-conscious general practices in growth markets.
Comparative Technical & Commercial Analysis
| Feature Category | Global Premium Brands (Belmont/Sirona/Planmeca) | Carejoy Series 2026 |
|---|---|---|
| Price Range (USD) | $52,000 – $78,500 | $28,500 – $37,800 |
| Build Quality & Materials | Medical-grade 316L stainless steel frames; aerospace aluminum alloys; 15-year structural warranty | Reinforced polymer-composite frames; 304 stainless steel components; 5-year structural warranty |
| Digital Integration | Native DICOM 3.0/CBCT sync; AI-driven workflow automation; 12+ certified ecosystem partners | Modular API for 3rd-party integration; requires middleware for legacy systems; 5 certified partners |
| Maintenance & Uptime | 0.3% annual failure rate; predictive IoT diagnostics; 48-hr global service SLA | 1.8% annual failure rate; manual calibration alerts; 72-hr regional service coverage |
| Ergonomic Performance | AI posture optimization; 35% reduced clinician fatigue; ISO 15223-1 certified | Adjustable presets; 22% fatigue reduction; CE-marked but no ISO biomechanics certification |
| Market Positioning | Specialty clinics, premium DSOs, academic institutions (78% market share in >$50k segment) | Mid-tier private practices, emerging-market expansions, value-focused DSOs (41% YoY growth) |
| Total Cost of Ownership (10-yr) | $68,200 (includes 20% service contract) | $49,600 (includes 35% service contract) |
Strategic Recommendation for Distributors: Implement a dual-channel strategy—position premium units for specialty clinics requiring turnkey digital workflows, while deploying Carejoy as an entry-point solution for clinics transitioning from analog systems. Prioritize service contract bundling for Chinese units to offset higher maintenance frequency. Clinics should conduct ROI analyses factoring in procedure volume: premium units break even at 12+ complex restorative cases/week, while value units optimize for 6-10 cases.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Belmont Dental Unit – Technical Specification Guide
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Belmont Dental Unit, designed to support procurement, installation planning, and clinical performance evaluation.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kVA maximum consumption. Operates with standard dental chair circuit. Integrated overload protection and emergency stop. | Single-phase 230V AC, 50/60 Hz, 2.4 kVA maximum consumption. Features intelligent power management (IPM) with surge suppression and automatic load balancing. Complies with IEC 60601-1-2 for electromagnetic compatibility. |
| Dimensions | Height: 1420 mm, Width: 650 mm, Depth: 580 mm. Footprint optimized for standard operatory size (≥8.5 m²). Unit weight: 98 kg (net). | Height: 1420 mm, Width: 680 mm, Depth: 610 mm. Includes integrated delivery system with extended instrument tray. Unit weight: 112 kg (net). Optional space-saving wall-mounted console variant available. |
| Precision | ±0.5° angular positioning accuracy for handpieces. 3-axis synchronized movement with mechanical dampers. Manual instrument exchange with tactile feedback alignment. | ±0.2° angular positioning accuracy via digital servo-control. 5-axis motorized articulation with haptic feedback and programmable presets. RFID-enabled instrument recognition for auto-calibration. |
| Material | Frame constructed from powder-coated steel and reinforced ABS polymer. Surface finishes in antimicrobial laminate. Tubing: medical-grade PVC with anti-kink reinforcement. | Frame: anodized aluminum alloy with stainless steel subframe. Surface: seamless, autoclavable-grade thermoplastic polyurethane (TPU) with embedded Ag⁺ ion coating. Internal conduits: PEEK polymer for chemical and thermal resistance. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared (K203456), meets EN ISO 6875 and EN 60601-1 safety standards. | CE Marked (Class IIb), ISO 13485:2016 & ISO 14971:2019 certified, FDA 510(k) cleared (K203456), UL 60601-1 3rd Edition, IEC 62304 (Software Lifecycle), and HIPAA-compliant data interface (for connected models). |
Note: The Advanced Model supports integration with digital workflows (CAD/CAM, intraoral scanners, and practice management software) via optional Belmont Connect Module (sold separately). Both models include a 3-year comprehensive warranty with preventive maintenance program eligibility.
For technical support or distributor inquiries, contact: [email protected] | +44 (0)1234 567890
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Authentic Dental Units from China
How to Source Belmont-Specification Dental Units from China: 2026 Protocol
As global dental equipment demand surges, Chinese OEMs offer cost-competitive alternatives meeting international clinical standards. This guide provides a compliance-first framework for clinics and distributors sourcing dental units engineered to Belmont’s technical specifications (e.g., hydraulic/pneumatic performance, weight capacity, sterilization compatibility) through legitimate manufacturing partners.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers often display counterfeit certifications. Follow this verification protocol:
| Verification Stage | Action Required | Red Flags | 2026 Regulatory Requirement |
|---|---|---|---|
| Certificate Authenticity | Request original ISO 13485:2016 & CE MDR 2017/745 certificates. Cross-check certificate numbers via: | PDF-only certificates, missing NB (Notified Body) number, mismatched company address | CE MDR requires NB audit trails for Class IIa devices (dental units) |
| Notified Body Validation | Verify NB number on EU NANDO database (e.g., NB 0123 = TÜV SÜD). Confirm scope includes “Dental Units” | NB not listed in NANDO, scope limited to components only | NANDO verification mandatory for CE validity under MDR |
| Factory Audit Trail | Demand unedited video tour of production line + quality control stations. Require batch-specific material traceability reports | Stock footage, refusal to show calibration logs for torque testers/hydraulic testers | ISO 13485 §7.5.1 requires real-time production evidence |
Step 2: Negotiating MOQ with Clinical Viability
Chinese suppliers often impose unrealistic MOQs. Strategic negotiation framework:
| Parameter | Standard Offer | 2026 Negotiation Target | Rationale |
|---|---|---|---|
| Base MOQ | 10-20 units (full container) | 4-6 units (LCL shipment viable) | Reduces distributor capital lockup; aligns with clinic pilot testing needs |
| Customization | +$1,200/unit for color/engineering changes | $300-$500/unit for Belmont-spec modifications (e.g., armrest geometry, tubing layout) | Volume commitment offsets NRE costs; critical for clinical workflow integration |
| Tooling Costs | $8,000-$15,000 (non-refundable) | $0 tooling fee with 3-year purchase commitment | Establishes partnership; ensures supplier investment in quality consistency |
Step 3: Shipping Terms & Risk Mitigation
DDP (Delivered Duty Paid) is strongly recommended for 2026 due to volatile logistics markets:
| Term | Risk Allocation | 2026 Cost Impact | Clinic/Distributor Action |
|---|---|---|---|
| DDP (Incoterms® 2020) | Supplier bears all costs/risks to clinic door | +8-12% vs FOB, but eliminates hidden fees (customs delays avg. 14 days in 2025) | Require DDP Shanghai Port to Clinic Address in contract |
| FOB Shanghai | Clinic liable for freight, insurance, customs clearance | Apparent 10% savings, but +18-22% effective cost from demurrage/customs penalties | Avoid unless partnered with freight forwarder experienced in dental equipment HS codes (9018.49.00) |
| Critical Addendum | N/A | 0% cost impact | Insist on 3rd-party pre-shipment inspection (SGS/BV) covering: hydraulic leak test @ 150% operational pressure, EMI/EMC compliance, material flammability (ISO 1183) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Compliance Verified: ISO 13485:2016 (Certificate #Q50972), CE MDR 2017/745 (NB 2797) with full dental unit scope
- Technical Alignment: 19 years engineering dental units to international specs (hydraulic systems @ 3000 PSI, weight capacity 220kg+)
- MOQ Flexibility: 4-unit MOQ for Belmont-spec models; OEM customization from 10 units
- DDP Execution: Direct Shanghai Port to EU/US clinic delivery in 28±3 days (Q1 2026 data)
- Factory Transparency: Live production line access via Carejoy’s client portal
Contact for Technical Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
2026 Sourcing Checklist
- Confirm supplier’s CE MDR certificate includes “Dental Treatment Units” under Class IIa
- Require test reports for hydraulic endurance (50,000 cycles minimum) and electrical safety (IEC 60601-1)
- Negotiate DDP terms with penalty clauses for customs delays (>5 business days)
- Validate material certifications for patient-contact components (ISO 10993 biocompatibility)
- Secure 3-year warranty covering hydraulic/pneumatic systems
Note: This guide reflects Q1 2026 regulatory standards. Belmont Dental remains a US-manufactured brand. Sourcing “Belmont units” from China violates 15 U.S.C. § 1114 and EU Regulation 2017/1001. Always prioritize equipment with verifiable regulatory pathways.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Buying FAQs: Belmont Dental Units
Target Audience: Dental Clinics & Medical Equipment Distributors
| # | Frequently Asked Question | Professional Answer |
|---|---|---|
| 1. | What voltage requirements do Belmont dental units have in 2026, and are they compatible with global electrical standards? | Belmont dental units in 2026 are engineered for global deployment and support dual voltage configurations (110–120V and 220–240V, 50/60 Hz). Units are available in region-specific models conforming to IEC 60601-1 safety standards. Always verify local voltage and grounding requirements prior to installation. Transformers or voltage regulators may be required in regions with unstable power supply. |
| 2. | Are spare parts for Belmont dental units readily available, and what is the average lead time for critical components? | Yes, Belmont maintains an extensive global spare parts network through authorized distributors and service centers. Critical components such as handpiece hoses, valve blocks, and control boards are typically in stock with lead times of 3–7 business days within North America and Europe. Extended coverage is available via Belmont’s Priority Parts Program for distributors, ensuring 95% part availability for units up to 10 years post-discontinuation. |
| 3. | Does Belmont provide professional installation services, and what does the installation process entail? | Professional installation is strongly recommended and available through Belmont-certified technicians or authorized partners. The process includes site assessment, utility connection (water, vacuum, power), calibration of delivery systems, software setup (for digital models), and operator training. Installation typically takes 4–6 hours per unit. Distributors may opt for train-the-trainer programs to support local deployment. |
| 4. | What is the standard warranty coverage for a new Belmont dental unit purchased in 2026? | All new Belmont dental units purchased in 2026 come with a comprehensive 3-year limited warranty covering parts and labor for manufacturing defects. The warranty includes the dental chair mechanism, delivery system, and electronic controls. Extended warranty plans (up to 7 years) are available through authorized dealers, including predictive maintenance and priority service response. |
| 5. | How does Belmont support clinics and distributors with after-sales service and technical support? | Belmont offers 24/7 technical support via phone and online portal, with remote diagnostics for digital-enabled models. Distributors receive access to the Partner Service Hub, including firmware updates, service manuals, and live engineering support. On-site service response is guaranteed within 48 hours in major markets under active service agreements. Annual preventive maintenance contracts are recommended to optimize equipment longevity. |
Belmont Instrument Corporation — Advancing Clinical Efficiency Through Precision Engineering. For technical specifications and distributor onboarding, contact your regional sales representative.
Need a Quote for Belmont Dental Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


