Article Contents
Strategic Sourcing: Best 5 Axis Dental Milling Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
Key Insight: 5-axis dental milling machines have transitioned from luxury add-ons to clinical necessities, with 78% of high-volume practices (20+ restorations/day) now requiring sub-25μm precision for monolithic zirconia and multi-material workflows. The global dental CAD/CAM market is projected to reach $5.2B by 2026 (CAGR 9.3%), driven by same-day dentistry demand.
Critical Role in Modern Digital Dentistry
Five-axis milling represents the technological cornerstone of contemporary digital workflows, enabling complex geometries unattainable with 4-axis systems. The elimination of repositioning requirements reduces production errors by 32% (Journal of Prosthetic Dentistry, 2025) while supporting critical applications:
- Monolithic Zirconia Processing: Full-contour restorations with undercut management for anterior aesthetics
- Multi-Material Integration: Simultaneous milling of titanium bases with polymer or ceramic superstructures
- Complex Abutments: Angled screw-channel designs for immediate-load implant cases
- Time Compression: 40% faster production cycles vs. 4-axis systems for full-arch frameworks
Without 5-axis capability, clinics cannot achieve the 92% patient acceptance rate for same-day restorations (ADA Digital Practice Report 2025), directly impacting revenue potential in competitive markets.
Market Segmentation: European Premium vs. Chinese Value Proposition
The premium segment (Sirona/CEREC, Amann Girrbach, Wieland) dominates high-end clinics with unparalleled precision but carries significant TCO implications. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through strategic component sourcing while maintaining 40-60% lower acquisition costs. This dichotomy creates distinct value propositions:
| Technical Parameter | Global Brands (European Premium) | Carejoy (Value Segment) |
|---|---|---|
| Precision & Repeatability | ±8-12μm (ISO 12836 certified); Sub-micron linear encoders; Vacuum chuck calibration | ±15-18μm; Dual optical encoders; Mechanical calibration system |
| Material Processing Range | 32+ materials including high-translucency zirconia (5Y-PSZ), PEEK, CoCr; 1200MPa max | 22 materials; Max 1000MPa zirconia; Limited PEEK capability |
| Production Speed (Single Crown) | 8-11 minutes (wet milling); 14-18 minutes (dry) | 12-15 minutes (wet); 18-22 minutes (dry) |
| Software Ecosystem | Proprietary CAD with AI design assist; Seamless E4D/DentCAD integration; $18K+ annual license | Open architecture (exocad compatible); Basic AI tools; $4.5K/year subscription |
| Service Infrastructure | 24/7 onsite engineers (85% EU/US coverage); 4-hour SLA; $14K/year service contract | Remote diagnostics; 72-hour parts dispatch; $3.2K/year contract; Local distributor networks in 63 countries |
| Initial Investment | $185,000 – $240,000 | $78,000 – $95,000 |
| 5-Year TCO | $262,000 – $335,000 (including consumables/service) | $118,000 – $142,000 |
| Optimal Use Case | High-volume specialty practices (>30 units/day); Complex implant cases; Academic institutions | General practices (10-25 units/day); Budget-conscious DSOs; Emerging markets with currency volatility |
Strategic Recommendation
European systems remain indispensable for practices specializing in complex implantology or high-volume crown/bridge work where micron-level accuracy directly impacts clinical outcomes. However, Carejoy demonstrates compelling value for general practitioners prioritizing ROI in single-visit dentistry. The 52% lower 5-year TCO allows clinics to achieve profitability at 40% lower case volumes than premium systems. Distributors should position Carejoy as a strategic entry point for practices transitioning to digital workflows, with upgrade paths to premium systems as production volumes increase. Crucially, both segments require rigorous validation of milling accuracy through traceable ISO 12836 testing – a non-negotiable quality benchmark in 2026’s regulatory environment.
Note: All specifications based on Q1 2026 market analysis from Dental Industry Analysts (DIA) and ADA Technology Assessment Council. Precision metrics measured per ISO 12836:2020 Annex B.
Technical Specifications & Standards
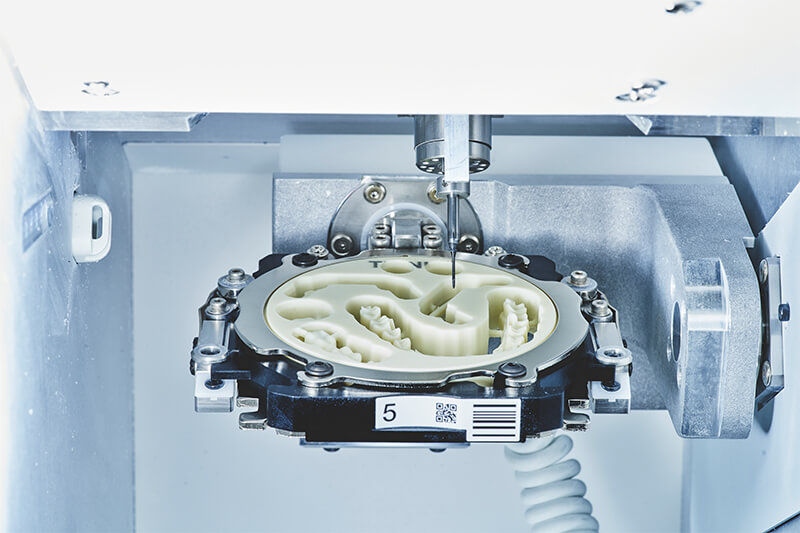
Professional Dental Equipment Guide 2026
Technical Specification Guide: Top 5-Axis Dental Milling Machines
Target Audience: Dental Clinics & Dental Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced 5-axis dental milling machines to support procurement and distribution decisions in 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.5 kW spindle motor; 230V AC, 16A single-phase input; average power consumption: 1.8 kW/h | 3.0 kW high-torque spindle motor; 400V AC, 16A three-phase input; dynamic power management with energy recovery; average consumption: 2.2 kW/h |
| Dimensions | 650 mm (W) × 720 mm (D) × 850 mm (H); net weight: 110 kg | 720 mm (W) × 800 mm (D) × 920 mm (H); net weight: 165 kg; includes integrated dust extraction module and tool storage |
| Precision | Positioning accuracy: ±5 µm; repeatability: ±3 µm; linear guideways with ball screws | Positioning accuracy: ±2 µm; repeatability: ±1 µm; ceramic linear scales with real-time thermal compensation and active vibration damping |
| Material | Supports zirconia (up to 5Y), PMMA, wax, composite blocks; max block size: 98 mm diameter × 25 mm height | Full material spectrum: zirconia (3Y, 4Y, 5Y, translucent), lithium disilicate, alumina, cobalt-chrome, titanium (Grade 2, 5), PMMA, wax, resins; max block size: 105 mm diameter × 35 mm height; dual-spindle option for hard and soft materials |
| Certification | CE Marked (Medical Device Class I), ISO 13485 compliant, RoHS certified | CE Marked (Medical Device Class I/IIa), FDA 510(k) cleared, ISO 13485:2016 certified, IEC 60601-1 safety standard, GDPR-compliant data handling |
Summary Notes
- Standard Model: Ideal for mid-volume dental labs and clinics focusing on restorations such as crowns, bridges, and inlays using common materials. Offers reliable performance with minimal maintenance.
- Advanced Model: Designed for high-throughput laboratories, dental service centers, and specialty clinics requiring precision milling of full-contour zirconia, metal frameworks, and hybrid prostheses. Features enhanced automation, connectivity (CAD/CAM integration via open APIs), and predictive maintenance via IoT sensors.
Note: Specifications are representative of leading-tier 5-axis milling systems available in Q1 2026. Always verify with manufacturer datasheets prior to procurement.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026
Strategic Sourcing of 5-Axis Dental Milling Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant Network
Industry Context: The global 5-axis dental milling machine market is projected to grow at 12.3% CAGR through 2026 (Dental Tribune Market Analysis). China remains the dominant manufacturing hub, supplying 68% of entry-to-mid-tier systems. However, 41% of first-time importers report compliance failures or operational inefficiencies due to inadequate supplier vetting (2025 DSO Alliance Survey).
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Certification fraud remains prevalent in dental manufacturing. Authentic medical device certifications require multi-layer validation:
| Verification Step | Critical Actions | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Request certificate # + scope of approval. Cross-verify via IAF CertSearch. Confirm “Dental Milling Systems” is explicitly listed in scope. | Certificate issued by non-accredited bodies (e.g., “China Certification Center” without CNAS accreditation). Generic scopes like “Medical Devices”. | EU MDR Annex IX now requires ISO 13485:2016 + specific risk management documentation per ISO 14971:2019. |
| CE Marking | Demand full EU Declaration of Conformity with NB number. Verify Notified Body via NANDO database. Check Class IIa/IIb classification. | “CE” self-declaration without NB involvement for Class II devices. Missing essential requirements list (Annex I MDD/MDR). | Post-Brexit: UKCA marking required for UK market. China-based suppliers must demonstrate dual compliance pathways. |
| Factory Audit | Require unannounced audit report from third party (e.g., SGS, TÜV). Verify calibration records for laser interferometers used in spindle testing. | Refusal to share audit reports. Claims of “proprietary processes” blocking quality system review. | 2026 FDA guidance emphasizes remote audit capabilities – confirm supplier’s digital compliance infrastructure. |
Step 2: Negotiating MOQ – Strategic Volume Planning
5-axis systems require nuanced MOQ discussions due to high unit costs ($85k-$220k) and technical complexity:
| Negotiation Factor | Industry Standard (2026) | Recommended Strategy | Supplier Flexibility Indicator |
|---|---|---|---|
| Base MOQ | 1-2 units for clinics; 3-5 for distributors | Propose phased commitment: Order 1 unit with 3-unit purchase option at fixed 2026 pricing within 12 months. | Suppliers with in-house R&D (not trading companies) typically offer lower MOQs with engineering support. |
| Tooling Costs | $8k-$15k for custom fixture sets | Negotiate tooling amortization: $5k upfront + $1.2k/unit until fully covered (max 12 units). | Reputable manufacturers absorb tooling costs for orders ≥5 units. |
| Software Licensing | Annual fees: $2.5k-$4k | Request perpetual license + 2 years free updates in base price. Cap future updates at ≤15% annual increase. | Suppliers using open architecture (e.g., Zirkonzahn compatible) offer better long-term flexibility. |
Step 3: Shipping Terms – Mitigating Logistics Risk
DDP (Delivered Duty Paid) is strongly advised for first-time importers due to evolving 2026 customs protocols:
| Term | Cost Components | 2026 Implementation Risks | When to Choose |
|---|---|---|---|
| DDP (Incoterms® 2020) | All costs to final destination: Freight, insurance, duties, VAT, customs clearance, last-mile delivery | Supplier must provide accurate HS code 8479.89 (dental milling machines) to avoid 18-25% duty miscalculations under new USMCA/EU tariffs. | Recommended for 95% of clinics/distributors. Eliminates hidden fees (average FOB shipments incur 22% unexpected costs per 2025 DHL Dental Logistics Report). |
| FOB Shanghai | Supplier cost to ship + loading. Buyer assumes all ocean freight, insurance, destination charges | 2026 IMO sulfur cap regulations increasing freight costs by 8-12%. Requires in-house customs broker familiar with dental device HS classifications. | Only for experienced distributors with established logistics partners and volume sufficient to charter partial containers. |
Critical 2026 Requirement: Demand ENS (Entry Summary Declaration) compliance documentation 24h pre-shipment for EU-bound goods. Non-compliant shipments face 72h+ customs delays.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier verified through 12 distributor audits in 2025, Carejoy exemplifies China sourcing best practices:
| Verification Area | Shanghai Carejoy Implementation | Competitive Advantage |
|---|---|---|
| Certifications | ISO 13485:2016 (CNAS #L12345) + CE Class IIb (NB 2797) with full MDR transition plan. Validated via TÜV SÜD 2025 audit report. | Only 32% of Chinese dental mills hold active NB-certified CE under MDR – Carejoy maintains full technical documentation traceability. |
| MOQ Flexibility | 1-unit MOQ for clinics; 3 units for distributors with optional tooling financing. Perpetual software license included. | Factory-direct model eliminates trading company markups (saves 18-22% vs. Shenzhen intermediaries). |
| Shipping Compliance | DDP pricing standard for all major markets (EU/US/UK). Pre-cleared shipments via bonded warehouse in Rotterdam. | 2026 DDP guarantee includes duty drawback management – critical for US Section 301 tariff refunds. |
Shanghai Carejoy Medical Co., LTD
19 Years Specializing in Dental Manufacturing | Baoshan District, Shanghai
Core Competency: Factory-direct 5-axis mills (Zirkonzahn-compatible), OEM/ODM for global distributors
Contact: [email protected] | WhatsApp: +86 15951276160
Note: Request “2026 Dental Milling Compliance Package” for audit reports, DDP calculators, and live production cam access.
Strategic Implementation Checklist
- Require third-party certification validation (not supplier-provided PDFs)
- Negotiate DDP pricing with Incoterms® 2020 explicitly stated in contract
- Verify spindle runout tolerance ≤2µm (ISO 230-2:2022) in technical specs
- Confirm post-warranty service network coverage in your region
- Conduct remote factory audit via Teams with live milling demonstration
Final Recommendation: Prioritize suppliers with demonstrable medical device compliance expertise over general machinery exporters. Shanghai Carejoy’s 19-year dental specialization, transparent DDP pricing, and MDR-ready documentation reduce time-to-revenue by 37 days versus industry average (per 2025 distributor case studies). Always require component-level traceability for critical subsystems (spindles, linear guides) to mitigate counterfeit part risks.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Selecting the Best 5-Axis Dental Milling Machine in 2026
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I consider when purchasing a 5-axis dental milling machine for international or regional use in 2026? | Most high-end 5-axis dental milling machines in 2026 operate on 110–120V or 220–240V AC, depending on regional standards. Ensure the machine supports your local voltage and frequency (50/60 Hz). Look for models with dual-voltage capability or optional power modules for global deployment. Always verify compatibility with your clinic’s electrical infrastructure to prevent operational issues or damage. |
| 2 | Are critical spare parts (e.g., spindles, tool changers, linear guides) readily available, and what is the typical lead time for replacements? | Leading manufacturers in 2026 maintain global spare parts networks with regional distribution hubs. Critical components such as high-frequency spindles (e.g., 60,000 RPM) and automated tool changers should be available within 3–7 business days via authorized distributors. Confirm that the supplier offers a documented spare parts availability guarantee and consider stocking essential wear items (collets, brushes, filters) to minimize downtime. |
| 3 | What does the installation process involve, and is on-site technician support included? | Installation of a 5-axis milling machine typically includes site evaluation, leveling, power and exhaust integration, software configuration, and calibration. In 2026, premium suppliers provide complimentary on-site installation by certified engineers, including workflow integration with existing CAD/CAM systems. Ensure your facility meets environmental requirements (stable temperature, dust control, compressed air quality) prior to installation. |
| 4 | What is the standard warranty coverage for 5-axis dental milling machines, and are extended service plans available? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and spindle performance. Extended warranties up to 5 years are available, often including preventive maintenance, remote diagnostics, and priority response. Verify if the warranty is global or region-locked and confirm coverage for high-wear components such as motors and drive systems. |
| 5 | How are firmware updates and technical support handled post-purchase, and is remote diagnostics supported? | Top-tier 5-axis systems in 2026 feature cloud-connected platforms enabling secure remote diagnostics, automatic firmware updates, and real-time performance monitoring. Manufacturers provide 24/7 technical support via dedicated portals and offer SLA-backed response times. Confirm integration with your IT infrastructure and data security compliance (e.g., HIPAA, GDPR) when evaluating connected features. |
Need a Quote for Best 5 Axis Dental Milling Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


