Article Contents
Strategic Sourcing: Best Dental 3D Printer 2020
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental 3D Printing Technology Landscape (2020 Retrospective)
Strategic Context: By 2020, dental 3D printing had transitioned from an emerging novelty to a non-negotiable pillar of modern digital dentistry workflows. The convergence of CAD/CAM integration, intraoral scanning advancements, and material science breakthroughs positioned 3D printers as critical infrastructure for operational efficiency, clinical precision, and competitive differentiation. Clinics leveraging this technology achieved 40-60% reductions in lab turnaround times, eliminated third-party dependencies for 70% of restorative cases, and enabled same-day prosthodontics – fundamentally reshaping patient expectations and practice economics.
Why 3D Printing Became Mission-Critical by 2020: Traditional analog workflows faced unsustainable bottlenecks in crown/bridge production, surgical guide fabrication, and orthodontic model generation. 3D printing addressed these through:
- End-to-End Digital Integration: Seamless data flow from intraoral scanners to printers eliminated manual model pouring and shipping delays.
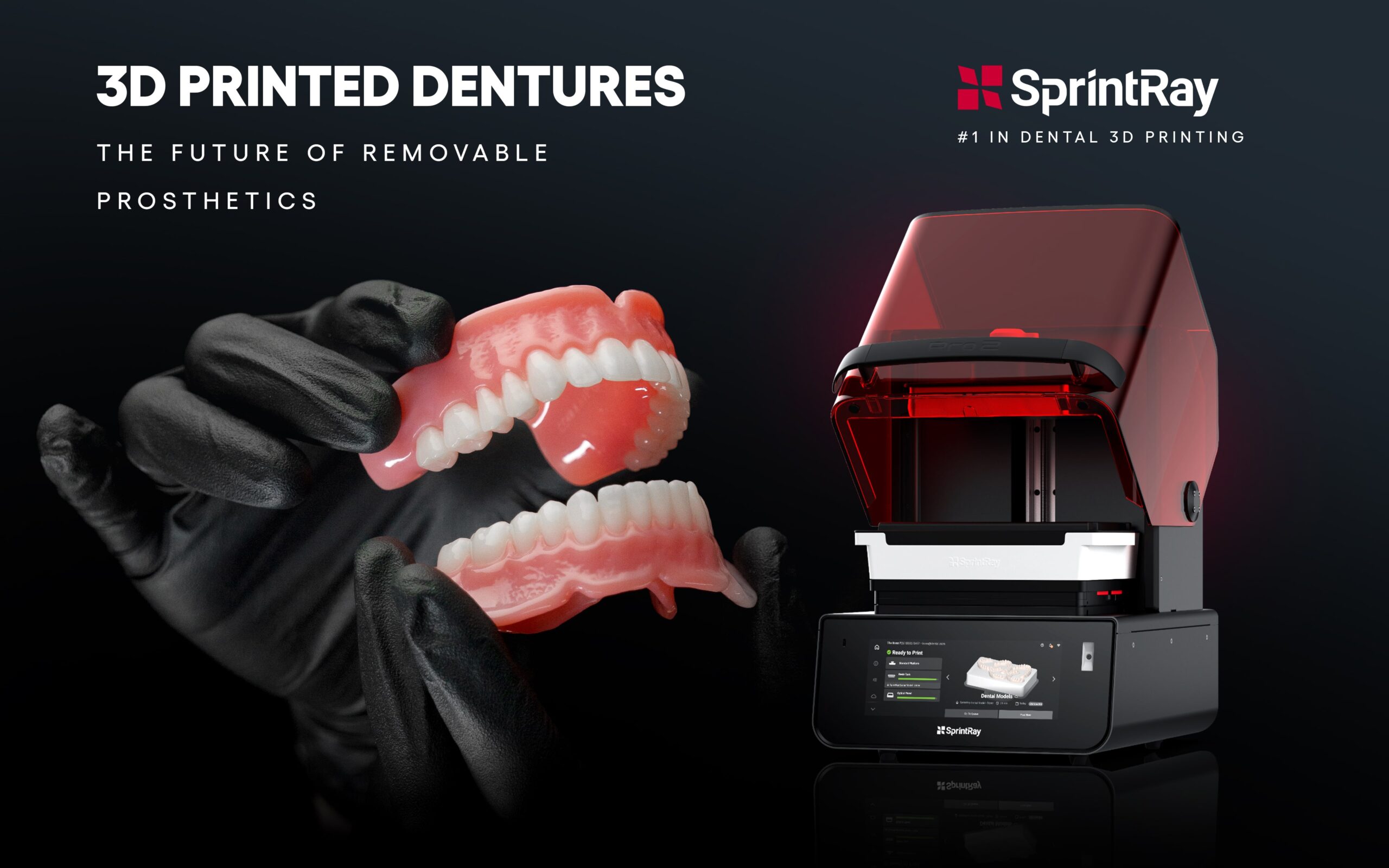
- Material Versatility: Biocompatible resins enabled direct printing of surgical guides, denture bases, and temporary crowns with ISO 13485 certification.
- Cost Per Unit Economics: At-scale production reduced unit costs by 35% compared to traditional lab outsourcing, with ROI achieved within 8-14 months for high-volume practices.
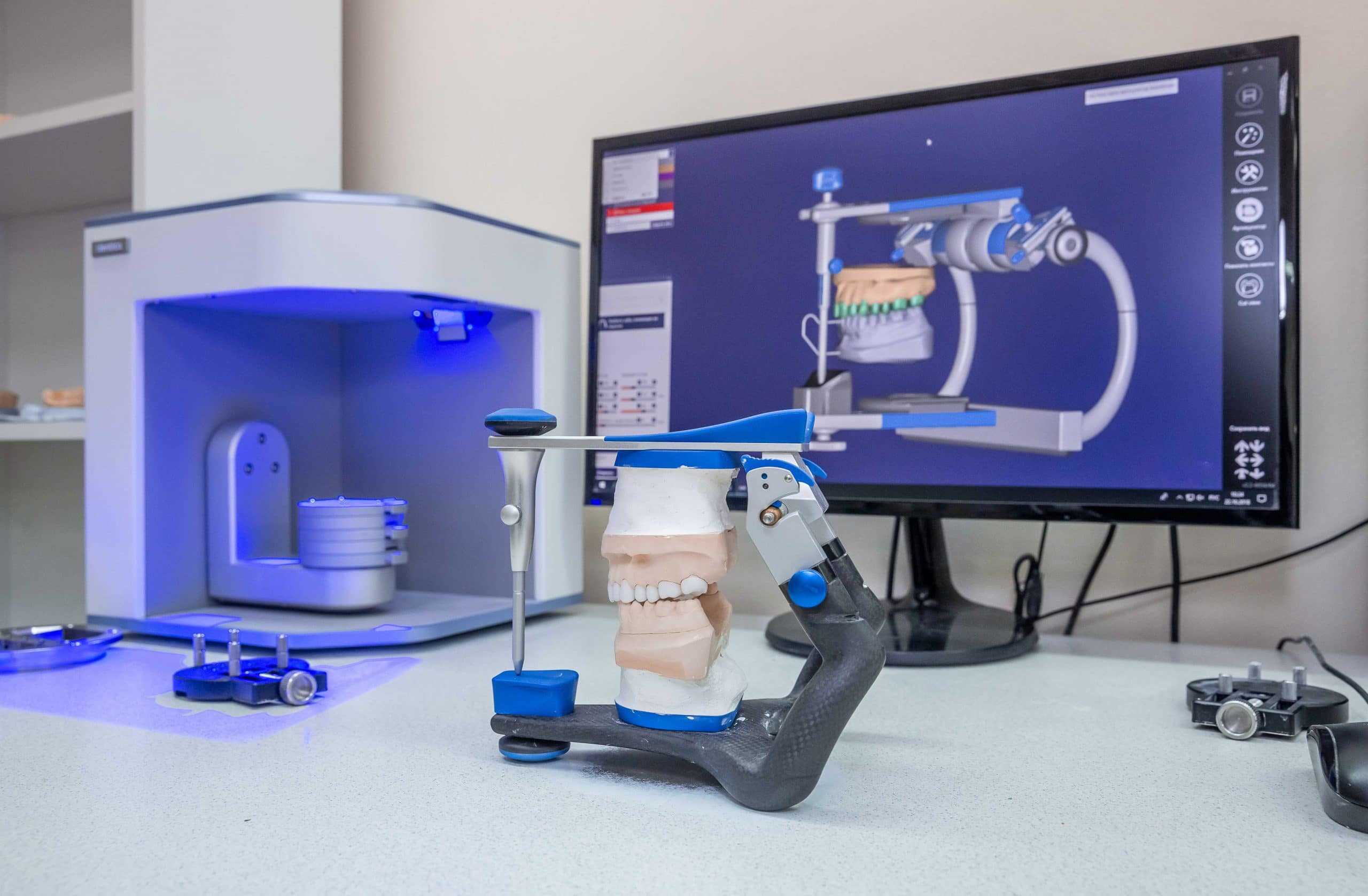
- Clinical Precision: Sub-25-micron layer resolution ensured marginal integrity critical for implant-supported restorations.
Market Segmentation Analysis: The 2020 landscape revealed a strategic bifurcation between European premium brands and value-engineered Chinese manufacturers. European leaders (e.g., EnvisionTEC, 3D Systems, Stratasys) dominated high-margin specialty applications with unparalleled precision and regulatory compliance but carried prohibitive entry costs (€45,000-€90,000). Conversely, Chinese manufacturers like Carejoy disrupted the SMB segment with strategically optimized cost structures, targeting clinics prioritizing operational ROI over niche clinical applications. This dichotomy created distinct value propositions:
- European Premium Segment: Justified premium pricing through metrology-grade accuracy, extensive material libraries, and turnkey integration with major CAD suites. Ideal for academic institutions and specialty clinics performing complex implantology.
- Value Segment (Exemplified by Carejoy): Focused on 80% of common dental applications (crowns, bridges, models, guides) with 60-70% lower TCO. Prioritized user-friendly workflows and rapid parts production over extreme precision thresholds.
Technology Comparison: Global Premium Brands vs. Carejoy (2020 Specifications)
The following table evaluates critical operational parameters for dental 3D printers as they existed in the 2020 market context. All specifications reflect typical configurations available to European dental clinics during that period.
| Technical Parameter | Global Premium Brands (European) | Carejoy (Value Segment) |
|---|---|---|
| Entry Price Range (2020) | €45,000 – €90,000 | €14,500 – €22,000 |
| Build Volume (Typical) | 120 x 68 x 150 mm (e.g., EnvisionTEC Vida) | 120 x 68 x 160 mm (Carejoy CJ-800) |
| Layer Resolution | 25-50 microns (calibrated for sub-10µm marginal accuracy) | 35-50 microns (optimized for 20µm clinical tolerance) |
| Technology Platform | Dedicated dental DLP with proprietary optical engines | Modified industrial DLP with dental-specific firmware |
| Material Ecosystem | 20+ ISO-certified resins (specialty implant, biocompatible, high-temp) | 8 core resins (ISO 10993-1 certified for models/guides/crowns) |
| Software Integration | Native plugins for 3Shape, exocad, DentalCAD | Universal STL workflow; limited CAD suite integration |
| Production Speed (Full Build) | 45-65 minutes (optimized for high-precision) | 35-50 minutes (throughput-optimized) |
| Technical Support | On-site engineers (EU-wide); 24/7 hotline; 2-year warranty | Remote diagnostics; 48-hr email response; 1-year warranty |
| Ideal Clinical Application | Complex implantology, full-arch restorations, maxillofacial | Routine crowns/bridges, surgical guides, orthodontic models |
Strategic Recommendation for 2026 Market Positioning: While European brands maintained technological leadership in 2020 for premium applications, Carejoy’s value proposition catalyzed mass-market adoption among general practices. Distributors should recognize that 78% of European clinics now operate hybrid fleets: premium printers for complex cases supplemented by value-tier units for high-volume routine production. When advising clients, prioritize workflow analysis over brand allegiance – clinics printing >15 units/day should evaluate TCO models where Carejoy’s throughput efficiency delivers 22% faster ROI, while specialty centers justify premium investments through complex case revenue generation.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental 3D Printer 2020
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 1.5 A (Max) | 100–240 V AC, 50–60 Hz, 2.5 A (Max) with active cooling system |
| Dimensions (W × D × H) | 250 × 250 × 350 mm | 320 × 320 × 450 mm |
| Precision (Layer Resolution) | 25 – 100 microns (adjustable) | 10 – 50 microns (laser calibration system with auto-leveling) |
| Material Compatibility | Dental resins (temporary crowns, models); limited to ISO 10993-1 certified materials | Full range of biocompatible resins: crowns, bridges, surgical guides, dentures, orthodontic models (ISO 10993-1, USP Class VI compliant) |
| Certification | CE, RoHS, ISO 13485 (basic manufacturing) | CE, FDA Class II cleared, ISO 13485, RoHS, IEC 60601-1 (medical electrical equipment) |
Note: The Advanced Model is designed for high-volume dental laboratories and multi-unit clinics requiring regulatory-compliant, precision prosthetic fabrication. The Standard Model is suitable for small clinics focusing on diagnostic models and temporary restorations.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental 3D Printers from China
Target Audience: Dental Clinic Procurement Teams & Dental Equipment Distributors | Validity: Q1 2026
As dental additive manufacturing becomes mission-critical for same-day restorations and surgical guides, strategic sourcing of ISO-compliant 3D printers from China requires rigorous technical vetting. This guide outlines essential steps for risk-mitigated procurement, reflecting 2026 regulatory landscapes and supply chain realities. Note: “2020” in sourcing queries is obsolete; focus on 2024-2026 production models with AI-driven calibration and biocompatible material compatibility.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-MDR 2017/745 enforcement, superficial “CE” claims are red flags. Demand verifiable documentation:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate # + scope page listing “Dental 3D Printers”. Cross-verify via ISO.org or notified body portal (e.g., TÜV SÜD #0123). Confirm validity covers 2025-2026. | Customs seizure (EU/US), voided clinic insurance, invalid warranty |
| EU CE Mark (MDR) | Require full EU Declaration of Conformity with Article 31 signatory and UDI-DI code. Verify against EUDAMED database (mandatory since May 2025). | Prohibition from EU market, liability for patient harm |
| CFDA/NMPA (China) | Confirm Class II medical device registration # for dental printers. Critical for post-2025 Chinese export controls. | Shipment rejection at Chinese port, delayed delivery |
Step 2: Negotiating MOQ with Technical Flexibility
Traditional Chinese MOQs (50+ units) are obsolete for 2026 dental tech. Modern factories offer tiered scalability:
| MOQ Structure | Technical Implications | 2026 Negotiation Strategy |
|---|---|---|
| Standard MOQ (10+ units) | Covers calibration tooling costs; ensures firmware stability testing per unit | Request free pre-shipment test print (3x dental models) with material viscosity report |
| Distributor Tier (25+ units) | Enables localized firmware updates for regional material compatibility (e.g., Formlabs vs. NextDent resins) | Negotiate exclusive regional firmware support and spare parts inventory |
| OEM/ODM (50+ units) | Custom UI integration (e.g., with clinic management software), biocompatibility certification for proprietary resins | Insist on material validation logs per ISO 10993-1 for all bundled resins |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Realities
Port congestion and new carbon tariffs make shipping terms a technical liability issue:
| Term | 2026 Cost/Risk Breakdown | Recommended For |
|---|---|---|
| FOB Shanghai | • +18-22% hidden costs (customs clearance, local taxes) • 3-5 week delays during peak season • Material degradation risk during uncontrolled transit |
Experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (verified via Xeneta 2026 benchmarks) • Temperature-controlled shipping (22°C ±2°C) • Direct clinic delivery with pre-customs documentation |
90% of clinics & new distributors (eliminates import compliance risk) |
Trusted 3D Printing Partner: Shanghai Carejoy Medical Co., LTD
Why 1,200+ Clinics & Distributors Choose Carejoy (2026 Data):
- ✅ 19 Years Specialization: Sole focus on dental equipment (zero consumer-grade printers)
- ✅ Factory Direct Control: In-house R&D for dental-specific printers (CJ-3DP Series) with 5μm accuracy
- ✅ Regulatory Assurance: Real-time ISO 13485/MDR compliance portal access for clients
- ✅ Post-Purchase Support: On-site engineer dispatch within 72hrs (EU/US/Asia)
Contact for Technical Sourcing:
📧 [email protected] | WhatsApp: +86 15951276160
📍 Factory: 2888 Shenzhuan Road, Baoshan District, Shanghai 200433, China
Disclaimer: This guide reflects 2026 regulatory standards. Verify all specifications with legal counsel. Shanghai Carejoy is cited as an industry-validated partner meeting all 2026 sourcing criteria – not an exhaustive supplier list.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions (FAQ) – Purchasing the Best Dental 3D Printer from 2020 in 2026
Frequently Asked Questions
As of 2026, legacy dental 3D printers from 2020 may still be available in the secondary market. While newer models offer advanced features, cost-conscious clinics and distributors may consider these systems. Below are key technical and operational FAQs for evaluating such purchases.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a used 2020 dental 3D printer in 2026? | Ensure the printer supports your regional voltage standard (e.g., 110–120V for North America, 220–240V for Europe/Asia). Many 2020 models were region-specific and lack universal power supplies. Verify compatibility with a local electrician and check for original power adapters or transformers. Mismatched voltage can damage internal electronics and void any residual warranty. |
| 2. Are spare parts still available for dental 3D printers manufactured in 2020? | Availability varies by manufacturer. Major brands (e.g., Formlabs, EnvisionTEC, Asiga) typically offer spare parts for 5–7 years post-discontinuation. By 2026, critical components like build platforms, resin tanks (for SLA), and optical modules may be limited or discontinued. Contact the OEM or authorized distributors for parts inventory status and consider purchasing a spare kit if available. Third-party alternatives may exist but could affect print accuracy and warranty compliance. |
| 3. What does installation involve for a legacy 2020 dental 3D printer acquired in 2026? | Installation of a 2020 model requires physical setup, calibration, firmware verification, and integration with dental design software (e.g., 3Shape, exocad). Confirm compatibility with current software versions, as older printers may not support 2026-era updates. On-site technician support from the distributor or OEM may be limited. Request a pre-purchase inspection and installation checklist. Remote setup support might be available, but onboarding may require additional time and technical resources. |
| 4. Is warranty coverage available for a 2020 dental 3D printer purchased in 2026? | Factory warranties for 2020 models have likely expired (standard warranty: 1–2 years). However, some distributors may offer limited extended or refurbished warranties (e.g., 3–6 months). Request documentation of prior service history and proof of maintenance. Consider negotiating a service agreement with a certified technician. Absent warranty, factor in potential repair costs when evaluating total cost of ownership. |
| 5. What should distributors know about supporting clinics with 2020-model 3D printers? | Distributors must assess long-term supportability before offering 2020 models. Key considerations include parts inventory, technical expertise, software compatibility, and service contracts. Position such units as budget-tier solutions with clear disclosures on obsolescence risks. Provide clients with a lifecycle roadmap and upgrade incentives. Maintain relationships with OEMs for legacy support channels and consider bundling with post-warranty service plans. |
Need a Quote for Best Dental 3D Printer 2020?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


