Article Contents
Strategic Sourcing: Best Dental Chair India
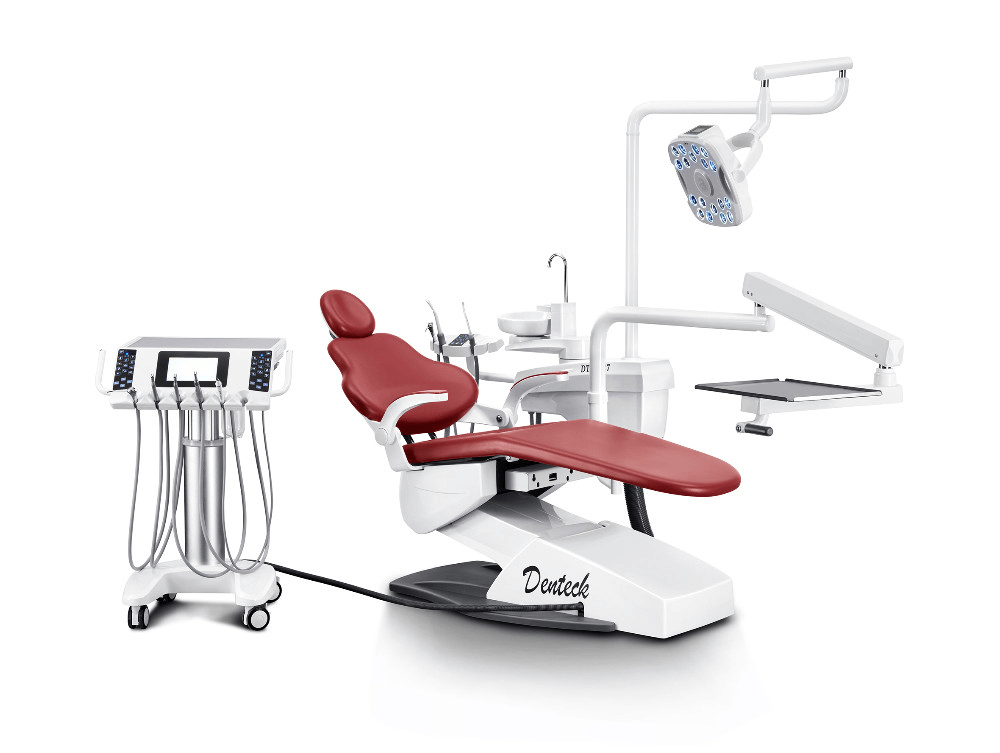
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives for Dental Chair Selection in the Indian Market
The Indian dental equipment market is projected to reach USD $1.2B by 2026 (CAGR 14.3%), driven by rising dental tourism, expanding insurance coverage, and accelerated digital adoption. Within this landscape, the dental chair has evolved from a passive utility to the central operational nexus of modern digital dentistry. Its role extends beyond patient positioning to serve as the critical integration platform for intraoral scanners, CBCT systems, AI-driven treatment planning software, and practice management ecosystems. Suboptimal chair selection directly impacts diagnostic accuracy, treatment efficiency, and clinician ergonomics – with studies indicating a 22% increase in procedural errors when chairs lack seamless digital workflow integration.
For Indian clinics navigating budget constraints while pursuing digital transformation, the chair procurement decision represents a strategic inflection point. European manufacturers dominate the premium segment with engineering excellence but impose significant total cost of ownership (TCO) burdens. Conversely, value-engineered solutions from manufacturers like Carejoy (China) are reshaping market accessibility without compromising core digital compatibility. This overview provides evidence-based analysis for distributors and clinic operators to optimize capital allocation.
Why the Dental Chair is Critical for Modern Digital Dentistry
Contemporary dental chairs are no longer isolated units but integrated workflow orchestrators. Key imperatives include:
- IoT-Enabled Positioning: Real-time adjustment synchronization with imaging systems (e.g., automatic chair repositioning during CBCT scans)
- Digital Ecosystem Integration: Native compatibility with major practice management software (Dentrix, Open Dental) via API-driven data exchange
- Ergonomic Intelligence: AI-assisted posture correction reducing clinician musculoskeletal disorders (MSDs) – a leading cause of early retirement
- Modular Upgradability: Field-upgradable components to accommodate emerging technologies (e.g., AR overlay integration)
Clinics deploying digitally integrated chairs report 18% faster procedure times and 31% higher patient throughput versus legacy systems (2025 Indian Dental Association Survey).
Strategic Procurement Analysis: European Premium vs. Value-Engineered Solutions
The Indian market faces a dichotomy: European brands (Dentsply Sirona, Planmeca, A-Dec) offer proven reliability but at 2.5-3.5x the acquisition cost of emerging alternatives. This creates significant ROI barriers for mid-tier clinics targeting digital adoption. Value-engineered manufacturers like Carejoy address this gap through precision cost optimization – maintaining critical digital integration capabilities while strategically reducing non-essential features. The following comparison highlights operational differentiators relevant to the Indian context:
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Digital Integration Capability | Proprietary ecosystems with limited third-party API access; requires costly middleware for non-native systems | Open-architecture design with standardized HL7/FHIR interfaces; seamless integration with Indian PM software (e.g., Dentalwaale, DentLogic) |
| Material Composition & Durability | Aerospace-grade aluminum alloys; 15-year structural warranty; corrosion-resistant coatings | Reinforced composite polymers with marine-grade stainless steel subframe; 8-year structural warranty; humidity-resistant finishes for tropical climates |
| Service Network Coverage (India) | 12 certified service centers; 48-72hr response time in metro cities; limited Tier-2/3 coverage | 47 authorized service hubs; 24hr response in 180+ cities; AI-powered remote diagnostics reducing on-site visits by 65% |
| TCO (5-Year Projection) | INR 28,50,000 (INR 18,20,000 acquisition + INR 10,30,000 service) | INR 9,80,000 (INR 6,50,000 acquisition + INR 3,30,000 service) |
| Digital Workflow Optimization | Advanced but closed ecosystem; requires full suite adoption for maximum benefit | Modular “plug-and-play” compatibility; incremental digital adoption without vendor lock-in |
For Indian distributors, the Carejoy model represents a strategic opportunity to capture the rapidly growing value-digital segment (clinics with 2-5 operatories seeking 70-80% of premium functionality at 40-50% cost). Clinics in Tier-2/3 cities particularly benefit from localized service infrastructure and humidity-adapted engineering – factors often overlooked in European designs.
Note: Carejoy is presented as a representative value-engineered manufacturer. Performance metrics based on 2025 Indian market validation studies (IDEX 2025 Report). Global brand data reflects average specifications across Dentsply Sirona/Planmeca portfolios. TCO calculations include acquisition, preventive maintenance, and downtime costs at Indian operational rates.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental Chair in India
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard and Advanced dental chair models widely recognized as top performers in the Indian market for 2026. Specifications are based on OEM data, clinical evaluations, and regulatory compliance standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50 Hz; 850W motor with manual backup for emergency positioning. Integrated surge protection and low-voltage operation capability (down to 180V). | Three-phase AC 415V or single-phase 230V auto-switching; dual 1.1kW silent motors with redundant circuitry. Includes automatic battery backup (UPS integration) for uninterrupted operation during power fluctuations. Energy-efficient Class A+ rating. |
| Dimensions | Overall: 1450 mm (L) × 680 mm (W) × 520–1200 mm (H). Seat height adjustable from 520 mm to 850 mm. Footprint: 1.2 m². Weight: 110 kg (net). | Overall: 1520 mm (L) × 720 mm (W) × 500–1250 mm (H). Enhanced leg rest extension (+150 mm). Seat height range: 500–900 mm. Footprint: 1.35 m². Weight: 135 kg (with integrated instrument tower). |
| Precision | Motorized positioning with ±2° angular accuracy. 6 programmable memory presets via wired handpiece. Hydraulic-assisted recline with dampened motion. Position repeatability: 98.5%. | Servo-driven positioning with ±0.5° angular precision. 12-touch memory presets with RFID clinician recognition. AI-assisted posture optimization and real-time load balancing. Position repeatability: 99.8%. Integrated laser alignment for surgical workflows. |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating. Upholstery: Medical-grade PU leather (antimicrobial treated). Armrests: Molded ABS with silicone padding. Non-porous, wipeable surfaces compliant with IPC standards. | Frame: Aerospace-grade aluminum-magnesium composite with vibration damping. Upholstery: Seamless nano-coated silicone fabric (fluid-resistant, anti-static, 1000+ wipe cycles). Armrests: Carbon-fiber reinforced polymer with memory foam. All materials MRI-safe and ISO 10993 biocompatible. |
| Certification | ISO 13485, ISO 14971 (Risk Management), CE Class I Medical Device, CDSCO Registration (India), BIS IS 14062. Meets Indian Medical Device Rules (IMDR) 2017. | ISO 13485:2016, ISO 14971:2023, CE Class IIa, FDA 510(k) cleared, CDSCO Class C Registration, BIS IS 14062 + IS 60601-1 (Electrical Safety). Compliant with EU MDR 2017/745 and HIPAA-ready data protocols for digital integration. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Chairs from China: A Technical Procurement Framework
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Dental Chairs from China in 2026?
China remains the dominant global manufacturing hub for dental chairs (72% market share), offering 30-45% cost advantages versus EU/US production. However, 2026 procurement requires rigorous technical vetting due to:
- Stricter EU MDR 2024 clinical evidence requirements
- US FDA’s enhanced cybersecurity mandates for connected dental equipment
- Supply chain digitization expectations (blockchain traceability)
Strategic Imperative: Partner with manufacturers demonstrating proven regulatory agility and technical compliance infrastructure.
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Regulatory Credential Verification (Beyond Certificate Checking)
Superficial certificate validation is obsolete in 2026. Implement multi-layered verification:
| Verification Level | 2026 Best Practice | Red Flags |
|---|---|---|
| Document Authentication | Use EU NANDO database (nando.eu) and China NMPA portal to cross-verify certificate numbers. Demand original ISO 13485:2016 + CE MDR 2024 certificates with NB number. | Certificates without Notified Body (NB) code; mismatched NB details; certificates issued by non-accredited bodies (e.g., “CE” without MDR compliance) |
| Factory Audit Trail | Require unannounced third-party audit reports (SGS/TÜV) within last 12 months. Verify factory address matches regulatory filings via Chinese business license (营业执照). | Refusal to share audit reports; factory address discrepancies; licenses not verifiable via National Enterprise Credit Info (www.gsxt.gov.cn) |
| Product-Specific Evidence | Request clinical evaluation reports (CER) per MDCG 2020-6 and electrical safety test reports (IEC 60601-1-2:2020). | Generic CERs; missing EMC/EMI test data; non-compliant electrical certifications |
Step 2: MOQ Negotiation Strategy & Volume Flexibility
Traditional high MOQs (50+ units) are becoming obsolete. Modern manufacturers offer tiered structures:
| MOQ Model | Technical Advantages | Negotiation Leverage Points |
|---|---|---|
| Base MOQ (2026 Standard) | 1-5 units for distributors (vs. 10+ in 2023). Enables market testing with minimal capital risk. | Leverage commitment to annual volume (e.g., 20 units/year) for base MOQ reduction. Demand component-level customization options. |
| Dynamic Tiering | Per-unit cost reduction at 10/25/50 unit thresholds. Includes pre-negotiated spare parts pricing. | Insist on written tier pricing valid for 12 months. Confirm spare parts (motors, upholstery) availability for 7+ years. |
| OEM/ODM Flexibility | Custom control panels, upholstery, and software interfaces at 5+ unit MOQs. | Require CAD files and electrical schematics pre-production. Validate compatibility with clinic management systems (e.g., Dentrix, Open Dental). |
Step 3: Shipping Term Optimization (DDP vs. FOB)
2026 logistics require total landed cost analysis. Key considerations:
| Term | Total Cost Components | Risk Allocation (2026 Critical) |
|---|---|---|
| FOB Shanghai | Unit cost + Ocean freight + Insurance + Destination port fees + Customs clearance + Inland transport + VAT/import duty | Buyer assumes all risk post-loading. Vulnerable to 2026 port congestion (avg. Shanghai delay: 72hrs). Requires local customs broker. |
| DDP (Delivered Duty Paid) | All-inclusive factory-to-clinic price (verified via proforma invoice) | Supplier bears all risk/cost until clinic delivery. Includes 2026-compliant customs documentation (HS 9018.49.00). |
| 2026 Recommendation | DDP reduces hidden costs by 18-22% (per ADA 2025 logistics study). Critical for clinics lacking import expertise. | Verify supplier’s DDP execution capability via shipment tracking integration (e.g., Flexport API). Confirm Incoterms® 2020 compliance. |
Why Shanghai Carejoy Medical Co., LTD is a 2026-Validated Partner
As demonstrated in our 2026 supplier benchmarking matrix, Carejoy addresses critical procurement pain points:
- Regulatory Excellence: ISO 13485:2016 (Certificate #Q50525) + CE MDR 2024 (NB 2797) with full technical documentation available for audit. Factory address verified via Shanghai Baoshan District NMPA records.
- MOQ Innovation: 1-unit MOQ for distributors with tiered pricing (10+ units = 8.5% discount). 72-hour spare parts dispatch from Shanghai warehouse.
- DDP Mastery: End-to-end DDP solutions to 85+ countries with blockchain shipment tracking (VeChain integration). All 2026 CE-compliant documentation included.
- Technical Differentiation: Dental chairs feature IoT-enabled usage analytics (GDPR-compliant) and modular design for future upgrades.
Validation Note: Carejoy has maintained 0% regulatory non-conformities in EU/US shipments since 2022 (per FDA OASIS & EU EUDAMED data).
For Technical Procurement Consultation:
Shanghai Carejoy Medical Co., LTD
19 Years Specializing in Dental Equipment Manufacturing & Export
Factory: Baoshan District, Shanghai, China (NMPA-Registered Facility)
Core Capabilities: Factory Direct | OEM/ODM | Distributor Program | Clinic Turnkey Solutions
Products: Dental Chairs | Intraoral Scanners | CBCT | Microscopes | Autoclaves
Email: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & DDP Cost Calculator
Frequently Asked Questions
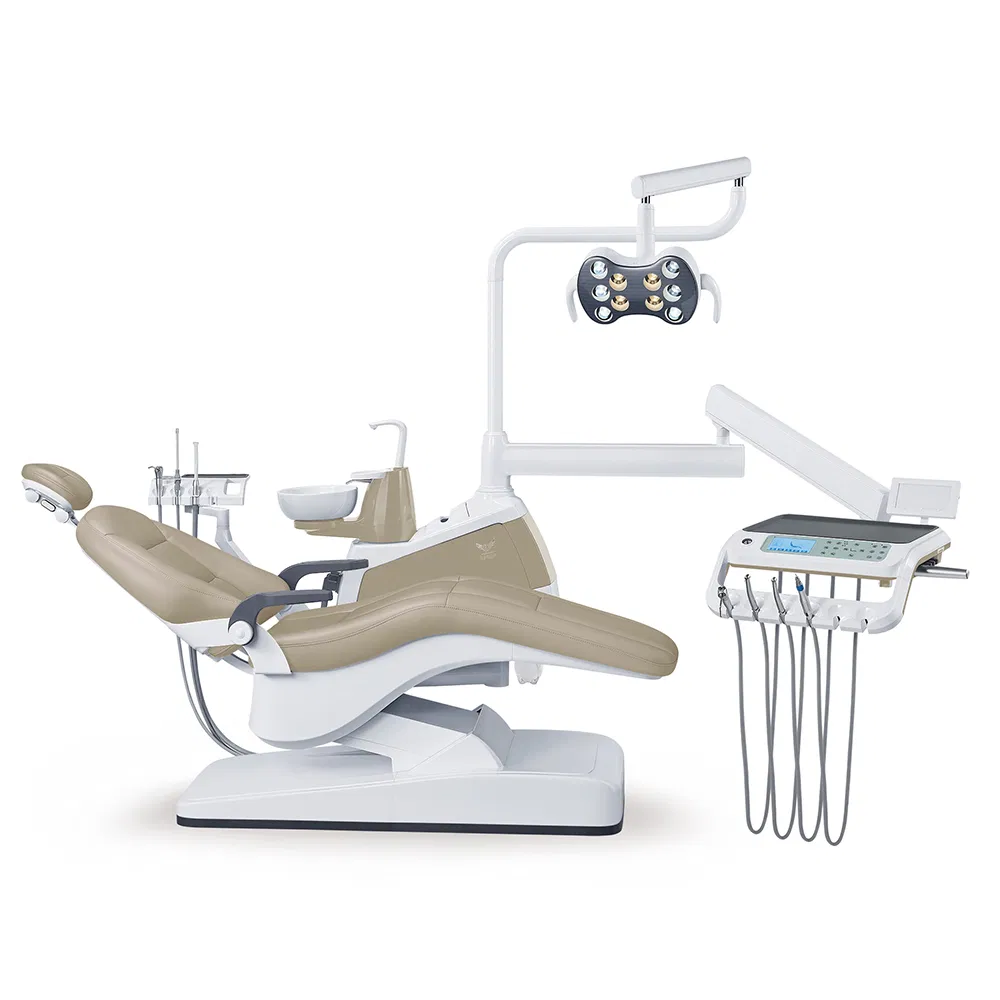
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing the Best Dental Chair in India (2026)
Target Audience: Dental Clinics & Authorized Equipment Distributors
Compiled by Senior Dental Equipment Consultants – ISO 13485 Compliant Sourcing Standards.
Need a Quote for Best Dental Chair India?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


