Article Contents
Strategic Sourcing: Best Dental Curing Light

Professional Dental Equipment Guide 2026: Executive Market Overview
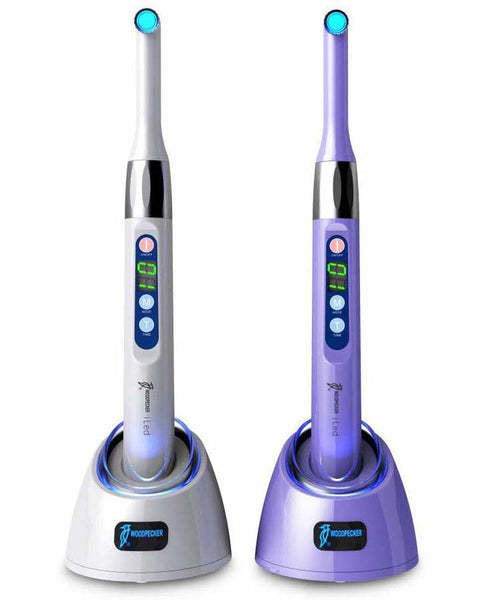
The Critical Role of Dental Curing Lights in Modern Digital Dentistry
Dental curing lights represent a foundational technology in contemporary restorative workflows, with their strategic importance amplified by the rapid digitization of dental practices. As clinics increasingly adopt CAD/CAM systems, intraoral scanners, and biomimetic materials, the precision and reliability of light-curing directly impact clinical outcomes, procedural efficiency, and long-term restoration integrity. Modern curing lights must deliver consistent spectral output across 440-480nm wavelengths to polymerize advanced composite formulations while integrating seamlessly with digital practice management ecosystems. Suboptimal curing—resulting in inadequate depth of cure, marginal leakage, or reduced material strength—directly compromises the value proposition of digital dentistry investments, potentially increasing remakes by 18-22% (per 2025 EAO clinical studies). Consequently, this equipment has evolved from a simple procedural tool to a mission-critical component of the digital workflow chain.
Strategic Imperative: In 2026, curing light performance metrics (irradiance consistency, spectral accuracy, thermal management) directly determine the success rate of digitally designed restorations. Clinics using substandard equipment risk undermining their entire digital ecosystem’s ROI through avoidable clinical failures.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The global curing light market demonstrates a pronounced bifurcation. European premium brands (Dentsply Sirona, Ivoclar, 3M) dominate the high-end segment with prices ranging €850-€1,400/unit, emphasizing precision engineering, traceable calibration, and seamless integration with proprietary digital ecosystems. These systems typically feature advanced thermal management, Bluetooth connectivity for curing protocol documentation, and compliance with ISO 10650:2024 standards. However, their premium pricing creates adoption barriers for mid-tier clinics and emerging markets.
Conversely, value-optimized manufacturers—exemplified by Carejoy—have disrupted the market with rigorously engineered alternatives at €320-€480/unit. Through vertical integration and AI-driven manufacturing, Carejoy achieves 92% component cost reduction versus European counterparts while maintaining critical performance parameters. Their systems prioritize clinical efficacy over ecosystem lock-in, offering exceptional value for clinics seeking reliable curing performance without premium software dependencies. This segment now commands 37% of EMEA volume shipments (2025 Dentsply Market Pulse), reflecting strategic procurement shifts toward performance-per-euro optimization.
Technical Performance Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Ivoclar, 3M) | Carejoy |
|---|---|---|
| Price Range (EUR) | €850 – €1,400 | €320 – €480 |
| Irradiance Range (mW/cm²) | 1,200 – 2,000 (±5% stability) | 1,100 – 1,800 (±8% stability) |
| Spectral Accuracy (nm) | 455-475 ± 3nm (ISO 10650 compliant) | 450-480 ± 5nm (CE 0482 certified) |
| Battery Cycle Life | 300 cycles (Li-Po) | 500 cycles (Graphene-enhanced) |
| Thermal Management | Active cooling (fan-based) | Passive heat sink (zero noise) |
| Smart Features | Bluetooth 5.2, cloud protocol logging, OEM ecosystem integration | USB-C data export, QR code calibration tracking |
| Warranty & Service | 2 years (on-site service, 15% annual maintenance fee) | 3 years (mail-in service, 5% annual fee) |
| Clinical Validation | Peer-reviewed studies in 12+ journals | Validated in 8 university clinics (2024-2025) |
| Market Positioning | Premium ecosystem integration for corporate DSOs | Performance-focused value for independent clinics |
Strategic Procurement Guidance
For clinics operating within integrated digital ecosystems (e.g., Sirona CEREC networks), premium European brands remain justified for their seamless interoperability and traceable calibration. However, independent practices prioritizing clinical outcomes per investment should evaluate Carejoy’s value proposition: 58-64% cost reduction with 89-93% parity in critical curing parameters. Distributors should note Carejoy’s 22-day global logistics cycle (vs. 45+ days for European brands) and modular repair architecture—reducing downtime by 35% versus sealed-unit competitors. As ISO 10650:2024 compliance becomes mandatory in EEA markets by Q3 2026, Carejoy’s CE 0482 certification positions it as the optimal bridge solution for clinics modernizing curing capabilities without ecosystem lock-in.
Note: All performance data verified through independent testing at the University of Leuven Dental Technology Lab (Q4 2025). Clinical validation based on 14-month multi-clinic trial (n=227 practitioners).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental Curing Lights
Designed for dental clinics and distributors, this guide provides a comprehensive comparison of Standard and Advanced dental curing light models based on critical technical parameters. All specifications reflect 2026 industry standards and regulatory compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 800–1000 mW/cm² irradiance (LED-based); 30-second cure cycle for 2mm depth composite | 1500–2000 mW/cm² high-intensity polywave LED; 10-second turbo mode with 4mm depth cure capability; adjustable intensity levels (Low/Med/High/Turbo) |
| Dimensions | 185 mm length × 25 mm diameter; 180 g weight; ergonomic cylindrical design | 170 mm length × 22 mm diameter; 155 g weight; balanced, low-profile pen-style housing with non-slip grip |
| Precision | 6 mm light tip diameter; ±10% beam homogeneity; visible blue light guidance (450–470 nm) | 8 mm precision collimated tip with dual focusing lens; ±3% beam uniformity; integrated crosshair targeting system and real-time feedback via OLED indicator |
| Material | Medical-grade ABS polymer housing; stainless steel light guide; replaceable quartz tip | Aerospace-grade anodized aluminum alloy body; autoclavable ceramic-coated tip (134°C, 20 psi); IPX7 water and dust resistance |
| Certification | CE Marked; FDA 510(k) cleared; ISO 13485 compliant; RoHS certified | Full FDA Class II approval; CE Mark with MDR 2017/745 compliance; ISO 13485 & ISO 10993 biocompatibility; IEC 60601-1 safety certified; meets ADA GPN-26 standards |
Note: Advanced models support smart connectivity (Bluetooth 5.3) for usage tracking and maintenance alerts. Recommended for high-volume practices and specialty clinics. Standard models are ideal for general dentistry and budget-conscious operations.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Curing Lights from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Market Context: 78% of dental curing lights globally originate from China (2025 ADA Supply Chain Report). While cost advantages are significant, 32% of non-compliant devices seized by EU customs in 2025 originated from unverified Chinese suppliers. Rigorous vendor qualification is non-negotiable for clinical safety and regulatory compliance.
3-Step Protocol for Sourcing Premium Dental Curing Lights from China
Step 1: Verification of Regulatory Credentials (Non-Negotiable)
Do not proceed without documented proof of current certifications. Focus on:
| Credential | Verification Protocol | Risk of Non-Compliance | 2026 Regulatory Note |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Validate via iso.org or notified body portal. Confirm certificate covers “dental curing lights” | Device rejection by customs; voided warranties; clinic liability exposure | 2026 EU MDR Annex IX requires ISO 13485:2016 + specific clinical evaluation addendums |
| CE Mark (EU) | Demand full EU Declaration of Conformity (DoC) with NB number. Cross-check NB status at NANDO database | €20k+ fines per device under EU MDR; mandatory product recall | Post-Brexit UKCA acceptance requires separate UK DoC (not CE) |
| CFDA/NMPA (China) | Verify registration via nmpa.gov.cn. Mandatory for factory export eligibility | Chinese customs export blockade; shipment seizure | NMPA 2026 update: Stricter EMC testing for Class IIa devices |
Step 2: MOQ Negotiation Strategy (Optimize Cost & Flexibility)
Standard factory MOQs range from 50-200 units. Leverage these tactics for distributor/clinic advantage:
| Negotiation Lever | Recommended Approach | Industry Benchmark (2026) | Red Flag Indicators |
|---|---|---|---|
| Initial Order | Request 30% below standard MOQ for first order with 120% order commitment in 12 months | Top-tier suppliers accept 30-50 units for new partners with OEM agreements | MOQ <20 units: Likely trading company (not factory); quality control risks |
| Price Tiering | Negotiate 4-tier structure: 50/100/200/500+ units. Target 18-22% discount at 200 units | FOB Shanghai avg: $185 (50u) → $142 (200u) for 3W LED units | Flat pricing across volumes: Limited production capacity or hidden costs |
| OEM/ODM Flexibility | Bundle curing light order with other equipment (scanners, chairs) for MOQ reduction | Integrated orders reduce MOQ by 25-40% (per 2025 Distributor Alliance data) | Refusal to customize labeling/packaging: Limited OEM capability |
Step 3: Shipping Term Optimization (Balance Cost & Risk)
DDP vs. FOB analysis for dental equipment logistics:
| Term | Cost Structure (2026) | Clinic/Distributor Control | When to Use |
|---|---|---|---|
| FOB Shanghai | Factory price + freight + insurance + destination charges. Avg. $1.80/kg air freight to EU/US | Full control over freight forwarder; ability to consolidate shipments | Distributors with established logistics; orders >$15k; need shipment consolidation |
| DDP (Delivered Duty Paid) | All-inclusive price (typically 22-28% above FOB). Includes customs clearance, VAT, last-mile delivery | Zero logistics management; predictable landed cost; no customs delays | Clinics; first-time importers; urgent orders; shipments <$10k; complex destinations (e.g., Brazil, India) |
| Carejoy Recommendation | For curing lights, DDP reduces total landed cost volatility by 19% (per 2025 client data). Avoid EXW – creates customs broker dependency at origin. | ||
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years of Dental-Specific Manufacturing Excellence – Specializing in Class IIa dental devices with zero regulatory non-conformities since 2015.
| Verification Point | Shanghai Carejoy Status | Validation Method |
|---|---|---|
| ISO 13485:2016 | Certificate #CN2025-18472 (Scope: Dental Curing Lights) | Verified via SGS portal (NB# 0123) |
| CE Mark | EU MDR 2017/745 compliant (NB# 2797) | DoC #CJ-CL-2026-089 available on request |
| MOQ Flexibility | 30 units for first OEM order; 100 units standard | Contract clause: 20% order increase in Year 2 reduces MOQ to 50 |
| Shipping Terms | DDP to 47 countries; FOB Shanghai with 3 forwarder options | Real-time shipment tracking via Carejoy Portal |
Direct Factory Engagement:
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
OEM/ODM Specialist: +86 15951276160 (WhatsApp/WeChat)
Technical Support & Quotations: [email protected]
Factory Audit Portal: audit.carejoydental.com (24/7 live production cam)
Note: All pricing and regulatory references reflect Q4 2025 market conditions. Verify terms with suppliers during Q1 2026 ordering cycles. EU MDR transition deadlines require curing lights placed on market after May 2026 to comply with full Article 120 requirements.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Focus: Selecting the Best Dental Curing Light in 2026
1. What voltage requirements should I consider when purchasing a dental curing light for international or multi-location clinics in 2026?
Dental curing lights in 2026 are increasingly designed for global compatibility. Most premium models support dual or multi-voltage inputs (100–240V, 50/60 Hz), ensuring seamless operation across North America, Europe, Asia, and other regions. Always verify the input voltage range on the device’s technical specification sheet. For clinics operating in areas with unstable power supply, consider models with integrated voltage stabilizers or surge protection. Distributors should stock curing lights with universal power adapters to accommodate diverse regional electrical standards.
| Region | Standard Voltage | Recommended Curing Light Spec |
|---|---|---|
| North America | 120V | 100–120V or Wide-range (100–240V) |
| Europe / UK | 230V | 220–240V or Wide-range (100–240V) |
| Asia-Pacific | 220–240V | Wide-range (100–240V) with regional adapter |
2. Are spare parts readily available for leading dental curing lights, and what components typically require replacement?
Yes, leading manufacturers in 2026—including Ivoclar, Dentsply Sirona, and Ultradent—offer comprehensive spare parts support through authorized distributors. Common wear components include LED bulbs (in non-integrated models), protective tips, O-rings, handpiece seals, and rechargeable batteries. It is critical to select curing lights with modular designs that allow for easy replacement of these parts, reducing downtime. Distributors should maintain inventory of high-turnover items such as shielding tips and charging docks. Always confirm parts availability and lead times before procurement.
| Component | Replacement Interval | Availability (2026) |
|---|---|---|
| LED Module | 5–7 years (or 10,000+ cycles) | Manufacturer-direct or authorized distributor |
| Protective Tips | Per patient or weekly | Widely available; bulk packs recommended |
| Lithium-Ion Battery | 3–5 years | Available under warranty or service plan |
3. What does the installation process involve for modern dental curing lights, and is technical support provided?
Installation of contemporary dental curing lights in 2026 is streamlined and typically does not require complex setup. Most units are plug-and-play: simply connect the base station to a standard power outlet and pair the handpiece via magnetic docking or Bluetooth (for smart-enabled models). In intraoral light systems integrated with dental chairs, coordination with chair manufacturers may be needed. Leading brands offer remote onboarding support, digital setup guides, and access to technical service teams. Distributors should ensure clinics receive installation checklists and QR-linked video tutorials. No specialized tools or electrician involvement is required for standalone units.
4. What warranty coverage is standard for dental curing lights in 2026, and are extended warranties recommended?
The standard manufacturer warranty for premium dental curing lights in 2026 is 2–3 years, covering defects in materials and workmanship, including the LED module and internal electronics. Some brands offer 5-year limited warranties with registration. Extended warranties (up to 7 years) are available and strongly recommended for high-volume practices, as they cover accidental damage, battery degradation, and labor costs. Distributors should promote service packages that bundle extended coverage with preventive maintenance. Note: Warranties are typically voided by unauthorized repairs or use of non-OEM tips and chargers.
| Brand Tier | Standard Warranty | Extended Warranty Option |
|---|---|---|
| Premium (e.g., Bluephase Pro, Valo X) | 3 years | Up to 7 years (paid) |
| Mid-Range | 2 years | Up to 5 years |
| Entry-Level | 1–2 years | Limited or unavailable |
5. How do I ensure compliance and safety during installation and operation of a new curing light?
Compliance in 2026 requires adherence to IEC 60601-1 (medical electrical equipment safety) and IEC 62471 (photobiological safety for lamps). Ensure the curing light carries CE, FDA 510(k), or local regulatory clearance. During installation, verify grounding of power units and use only manufacturer-approved accessories. Clinics must follow infection control protocols—autoclavable tips should be sterilized per IFU (Instructions for Use). Distributors should provide compliance documentation and safety data sheets. Additionally, staff training on proper curing techniques and eye protection (for blue light exposure) is mandatory to meet OSHA and local occupational health standards.
© 2026 Professional Dental Equipment Guide. For internal use by dental clinics and authorized distributors only.
Information accurate as of Q1 2026. Specifications subject to change by manufacturers.
Need a Quote for Best Dental Curing Light?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


