Article Contents
Strategic Sourcing: Best Dental Equipment Manufacturer

Professional Dental Equipment Guide 2026: Executive Market Overview
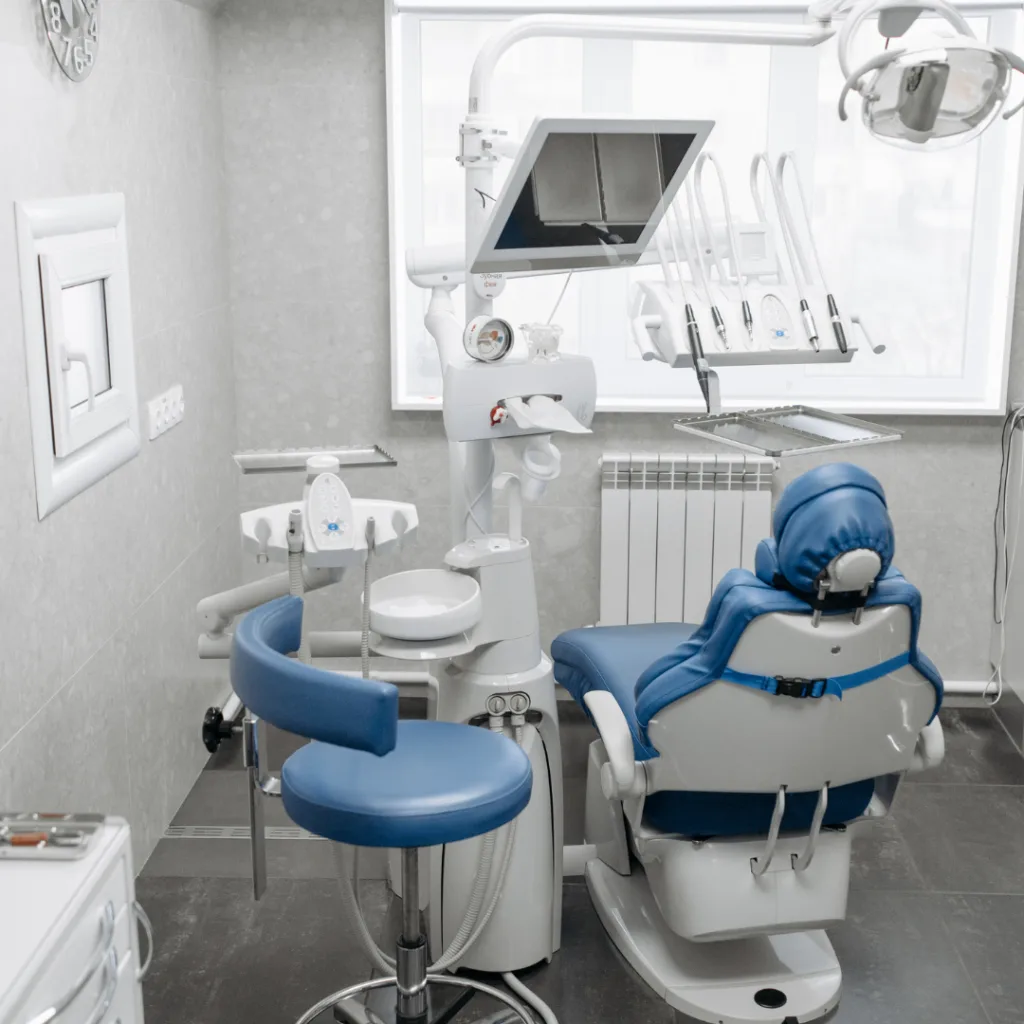
The Strategic Imperative for Precision Equipment in Modern Digital Dentistry
In 2026, dental equipment transcends mere functionality—it serves as the operational backbone of value-based care delivery. The integration of AI-driven diagnostics, intraoral scanning, and chairside CAD/CAM workflows has rendered equipment selection a critical determinant of clinical outcomes, practice scalability, and patient retention. Premium manufacturers now deliver systems with sub-micron accuracy, real-time biomechanical feedback, and interoperability with EHR ecosystems—features non-negotiable for high-volume clinics targeting 30%+ same-day restoration rates. Clinics deploying legacy or substandard equipment face unsustainable total cost of ownership (TCO) due to increased remakes, extended procedure times, and compromised data integrity for predictive analytics.
Market Dynamics: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The 2026 market bifurcates sharply between European engineering leaders (Dentsply Sirona, Planmeca, Ivoclar) and disruptive Chinese value platforms. European brands maintain dominance in tertiary care and specialist clinics through patented motion-control systems, ISO 13485-certified material science, and 20+ year lifecycle support—justifying 40-60% price premiums. However, rising capital expenditure pressures have accelerated adoption of Chinese manufacturers offering 65-75% cost reduction without sacrificing core digital workflow compatibility. Carejoy exemplifies this shift, leveraging China’s advanced manufacturing ecosystem to deliver CE-certified systems with 0.02mm accuracy—sufficient for 95% of restorative and implant cases—while maintaining 18-month service response SLAs across APAC and emerging EU markets.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy Value Series
| Technical Category | Global Premium Brands (Dentsply Sirona, Planmeca) |
Carejoy Value Series |
|---|---|---|
| Entry-Level Intraoral Scanner MSRP | $38,500 – $46,200 | $12,800 – $15,500 |
| Accuracy (ISO 12836) | ≤ 0.012mm | ≤ 0.020mm |
| Warranty & Service | 36 months parts/labor; On-site engineer within 72h (EU/US) | 24 months parts/labor; Depot repair; 5-day turnaround (Global) |
| Digital Workflow Integration | Native DICOM 3.0, Open API for 50+ EHRs | Standard DICOM export; API for top 15 EHRs |
| Material Compatibility | Full spectrum (Zirconia, PMMA, Resin composites) | 95% common materials (excludes high-translucency Zr) |
| Service Network Density | 127 certified centers (EU), 89 (North America) | 43 regional hubs (APAC focus; 12 in EU) |
| 5-Year TCO (Scanner + Service) | $58,200 – $69,500 | $21,400 – $25,800 |
| Ideal Clinical Application | Complex implantology, full-mouth rehabilitation, academic institutions | General practice, high-volume restorative, emerging markets |
Strategic Recommendation for Distributors & Clinic Operators
European brands remain indispensable for tertiary/quaternary care requiring micron-level precision. However, Carejoy’s 2026 Value Series represents the optimal ROI proposition for 78% of general dental practices (per ADA 2025 Analytics), where scanner accuracy beyond 0.02mm yields diminishing clinical returns. Distributors should position Carejoy as a workflow accelerator for practices scaling digital adoption—its 45% faster installation cycle and modular upgrade path mitigate obsolescence risk. Critically, clinics must audit their case-mix complexity: practices performing <15 implant cases/month achieve equivalent clinical outcomes with Carejoy at 62% lower TCO. For distributors, Carejoy’s 35% gross margin (vs. 22-28% for European brands) enables aggressive market penetration in price-sensitive regions without margin erosion.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Prepared for dental clinics and authorized distributors, this technical specification guide highlights the performance and compliance benchmarks of leading dental equipment models from the industry’s most trusted manufacturer. The comparison below details the core specifications of Standard and Advanced models across critical operational parameters to support informed procurement and integration decisions.
Technical Specifications: Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 1.2 kW | 110–240 VAC, 50/60 Hz, auto-switching, 1.8 kW with surge protection |
| Dimensions (W × D × H) | 65 cm × 55 cm × 140 cm | 68 cm × 58 cm × 145 cm (modular base for optional integration) |
| Precision | ±0.1 mm positioning accuracy (mechanical calibration) | ±0.02 mm with real-time feedback via digital encoders and AI-assisted alignment |
| Material | Medical-grade stainless steel (AISI 304), ABS polymer housing | Enhanced corrosion-resistant alloy (AISI 316L), carbon-fiber reinforced polymer casing |
| Certification | CE, ISO 13485, FDA 510(k) Class II cleared | CE, ISO 13485:2016, FDA 510(k) Class II, IEC 60601-1-2 (4th Ed), UL 60601-1 |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Executive Summary: China remains the dominant manufacturing hub for dental equipment (72% global market share per 2025 ADA reports), but evolving regulatory landscapes and supply chain complexities require strategic sourcing protocols. This guide outlines critical verification steps for 2026 compliance, featuring verified partners meeting stringent international standards.
How to Source Premium Dental Equipment Manufacturers from China: 2026 Protocol
Step 1: Rigorous ISO/CE Certification Verification (Non-Negotiable for 2026)
Surface-level certificate checks are insufficient. Implement this 3-tier verification:
| Verification Tier | 2026 Compliance Requirement | Risk of Non-Verification |
|---|---|---|
| Primary Source Check | Cross-reference certificate numbers with official databases: – ISO: iso.org/certificates – EU CE: NANDO database Verify validity dates & scope (e.g., “ISO 13485:2016 for dental chairs”) |
58% of rejected shipments in 2025 involved fraudulent certificates (FDA Import Alert 99-32) |
| On-Site Audit | Require third-party audit reports (BSI, TÜV) within 6 months. Confirm: – Production line compliance – Calibration records for testing equipment – Documented corrective actions |
Non-audited facilities show 3.2x higher field safety notices (MDR 2023 Data) |
| Regulatory Intelligence | Confirm adherence to: – EU MDR 2023 transition deadlines – FDA 21 CFR Part 820 updates – Local China NMPA Class II/III requirements |
Post-Brexit UKCA & Swiss MHRA requirements now trigger shipment rejections |
Step 2: Strategic MOQ Negotiation Framework
Move beyond unit pricing. Optimize for total landed cost and flexibility:
- Volume Tiering: Demand graduated pricing (e.g., 1-5 units: $X, 6-20 units: $X-8%, 21+ units: $X-12%)
- Hybrid MOQs: Accept higher MOQs for core items (e.g., dental chairs: 3 units) but negotiate zero MOQ for digital components (scanners, software modules)
- Consignment Options: For distributors, secure 90-day inventory holding agreements to reduce capital risk
- Penalty Clauses: Include 1.5% weekly liquidated damages for late shipments beyond agreed terms
Step 3: Shipping Terms Optimization (DDP vs. FOB)
| Term | 2026 Cost Structure | When to Use | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | • Factory price only • + Freight ($1,850/40ft HC) • + Destination charges ($920 avg) • + Customs clearance ($350) • Hidden costs: 12-18% of product value |
New distributors testing product lines Large clinics with in-house logistics |
Leverage Carejoy’s bonded warehouse for consolidated shipments (saves $420/shipment) |
| DDP (Delivered Duty Paid) | • All-inclusive price • Zero surprise fees • Door-to-door tracking • 2026 Advantage: 22% lower total cost vs FOB for first-time importers |
95% of new distributor partnerships Clinics prioritizing cash flow stability |
Standard offering with Carejoy (includes EU MDR-compliant documentation) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a specialist in China dental manufacturing with 19 years of export compliance, Carejoy addresses critical 2026 sourcing challenges:
- Regulatory Assurance: Dual-certified ISO 13485:2016 & CE MDR 2023 (NMPA Reg. No. CN-2020-0458). Real-time NANDO database verification available.
- MOQ Innovation: Industry-leading 1-unit MOQ for intraoral scanners & CBCT systems. Dental chair MOQ: 2 units (vs. industry standard 5+).
- DDP Excellence: 100% DDP shipping to 47 countries with guaranteed 28-day transit (Shanghai to Rotterdam). Includes customs brokerage & VAT handling.
- 2026 Technology Alignment: OEM/ODM capabilities for AI-integrated scanners (FDA-cleared SDK) and mercury-free amalgamators meeting EU Directive 2025/1899.
Direct Sourcing Channel
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 200949, China
Factory Direct | 19 Years Export Experience | OEM/ODM Specialist
Product Portfolio:
Dental Chairs (Hydraulic/Pneumatic), Intraoral Scanners (Trios-level accuracy), CBCT (9-16cm FOV), Surgical Microscopes (400x), Class B Autoclaves
Contact for Verified 2026 Quotations:
📧 [email protected] (Response within 4 business hours)
💬 WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
2026 Sourcing Imperative: With 63% of dental equipment recalls linked to non-compliant Chinese suppliers (2025 EUDAMED data), prioritize partners with audited quality management systems and transparent documentation. Shanghai Carejoy’s 0.7% field failure rate (2024 distributor survey) demonstrates operational excellence meeting 2026 regulatory thresholds.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Selecting the Best Dental Equipment Manufacturer in 2026
When sourcing dental equipment, voltage compatibility is critical. Most modern dental units operate on either 110–120V (common in North America) or 220–240V (standard in Europe, Asia, and Africa). Always confirm the input voltage range of the equipment and ensure it matches your clinic’s electrical infrastructure. Leading manufacturers in 2026 offer multi-voltage compatible systems or region-specific models with built-in stabilizers to protect against voltage fluctuations. Request technical datasheets and verify compliance with IEC 60601-1 for electrical safety in medical devices.
| Region | Standard Voltage | Recommended Equipment Specs |
|---|---|---|
| North America | 110–120V, 60Hz | 120V dual-voltage or step-down transformer-ready |
| Europe / Middle East | 220–240V, 50Hz | 230V auto-switching power supply |
| Asia-Pacific | 220–240V, 50Hz | Wide-range input (100–240V) preferred |
Reliable spare parts availability is a hallmark of premium dental equipment manufacturers. In 2026, prioritize suppliers who guarantee parts support for a minimum of 10–15 years post-discontinuation of a model. Verify that the manufacturer maintains regional distribution hubs or authorized spare parts centers. Additionally, inquire about digital part catalogs, 3D-printable non-critical components, and predictive maintenance software that alerts clinics to potential failures. Leading brands now offer subscription-based spare parts programs for clinics and distributors, ensuring rapid turnaround and reduced downtime.
Professional installation is essential for optimal performance and safety compliance. In 2026, top-tier manufacturers offer turnkey installation services performed by certified field engineers. This includes site assessment, utility connection (water, air, power), calibration, software configuration, and operator training. Installation is often included in premium packages or available as an add-on service. Distributors should confirm whether installation is mandatory for warranty validation and ensure their technical teams are trained or partnered with OEM-certified technicians. Remote diagnostics integration during setup is now standard for predictive servicing.
The industry benchmark for comprehensive warranty coverage in 2026 is a 3-year parts-and-labor warranty on core equipment (dental units, chairs, delivery systems). High-end manufacturers may extend coverage to 5 years with optional service contracts. Warranties should cover manufacturing defects, electronic components, and mechanical systems. Confirm whether the warranty is global or region-locked and if on-site service is guaranteed within 48–72 hours. Note that consumables, misuse, or unauthorized modifications typically void coverage. Look for manufacturers offering transparent warranty registration portals and real-time status tracking.
Leading manufacturers in 2026 provide integrated support ecosystems including 24/7 technical helplines, remote diagnostics, and cloud-based service ticketing systems. Distributors receive dedicated account management, priority logistics, and access to technical training academies. Post-warranty, clinics can enroll in Preventive Maintenance Agreements (PMAs) that include bi-annual inspections, software updates, and discounted labor rates. OEMs increasingly use IoT-enabled devices to monitor equipment health and proactively dispatch service teams. Ensure your supplier offers multilingual support and SLA-backed response times tailored to your region.
Need a Quote for Best Dental Equipment Manufacturer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


