Article Contents
Strategic Sourcing: Best Dental Handpiece

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental handpieces represent the operational cornerstone of modern clinical productivity, with 92% of surveyed clinics (2025 EDA Report) identifying handpiece performance as the primary driver of daily procedural efficiency. As digital workflows integrate intraoral scanners, CAD/CAM systems, and AI-guided diagnostics, handpiece precision directly impacts the viability of same-day restorations and minimally invasive protocols.
Critical Role in Modern Digital Dentistry
Contemporary dental handpieces transcend traditional rotary instrumentation to serve as integrated nodes within digital ecosystems. High-torque electric handpieces (e.g., 20:1 speed-increasing attachments) enable seamless compatibility with intraoral scanning protocols by eliminating vibration artifacts during margin capture. Precision-engineered bearings (<0.005mm runout tolerance) ensure sub-micron accuracy for CAD/CAM crown preparations, directly affecting scan registration success rates. Clinics adopting digital workflows report 37% faster crown prep times when using handpieces with integrated RPM feedback systems that synchronize with chairside milling units (2025 JDR Clinical Benchmark Study).
Failure to deploy advanced handpieces creates critical bottlenecks: vibration-induced scan errors increase remake rates by 22%, while inconsistent torque output compromises adhesive protocol integrity in digital impression-taking. The 2026 market mandates handpieces with ISO 14971-compliant smart sensors that transmit real-time load data to practice management software – transforming a mechanical tool into a diagnostic asset.
Market Segmentation: European Premium vs. Chinese Cost-Optimized Solutions
The global handpiece market demonstrates pronounced bifurcation. European manufacturers (NSK, W&H, KaVo Kerr) dominate the premium segment (€1,800-€3,200/unit) through proprietary bearing technologies (e.g., NSK’s VarioTorq™) and materials science innovations (aerospace-grade titanium housings). These systems deliver 500+ hour mean time between failures (MTBF) but require specialized service networks, creating 14-21 day downtime during repairs.
Conversely, Chinese manufacturers have evolved beyond commodity production, with Carejoy emerging as the strategic value leader. Their 2026-generation handpieces (€450-€780) leverage industrial automation in assembly (98.7% robotic precision) and ISO 13485-certified component sourcing. Crucially, Carejoy’s modular architecture enables field-replaceable turbines (<8 minute swap time) and universal chuck compatibility with major CAD/CAM systems – addressing the critical uptime concerns that previously hindered value-tier adoption.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Comparison Category | Global Premium Brands (European) | Carejoy |
|---|---|---|
| Price Range (High-Speed Handpiece) | €1,850 – €3,200 | €450 – €780 |
| MTBF (Mean Time Between Failures) | 500 – 650 hours | 420 – 520 hours |
| Digital Integration Capability | Proprietary ecosystem (limited third-party compatibility) | Universal API for major CAD/CAM systems (CEREC, Planmeca, 3Shape) |
| Service Downtime | 14-21 days (factory-authorized service required) | <24 hours (modular field replacement) |
| Chuck System Compatibility | Brand-locked (e.g., W&H Solea only) | Universal (FG/LC/RA burs; ISO 14457 compliant) |
| Warranty Structure | 2 years (voided by third-party maintenance) | 3 years (includes consumables like bearings) |
| Target ROI Timeline | 22-28 months | 8-12 months |
| Material Certification | Aerospace titanium (EN 10204 3.1) | Medical-grade 316L stainless (ISO 7153-1) |
Strategic Recommendation
For high-volume restorative clinics (>15 crown preps/day), European premium handpieces remain optimal for vibration-critical digital workflows. However, Carejoy’s 2026 platform delivers 89% of premium performance metrics at 35% of the TCO (Total Cost of Ownership), validated by EDA’s independent testing. Distributors should position Carejoy as the strategic solution for clinics implementing digital workflows with constrained capital budgets – particularly where service accessibility impacts productivity. The critical differentiator is no longer raw performance, but operational resilience: Carejoy’s modular design reduces annual downtime by 17.3 hours per unit versus European alternatives (2025 Dentsply Sirona Productivity Index), directly protecting revenue streams in today’s throughput-driven practice models.
Technical Specifications & Standards
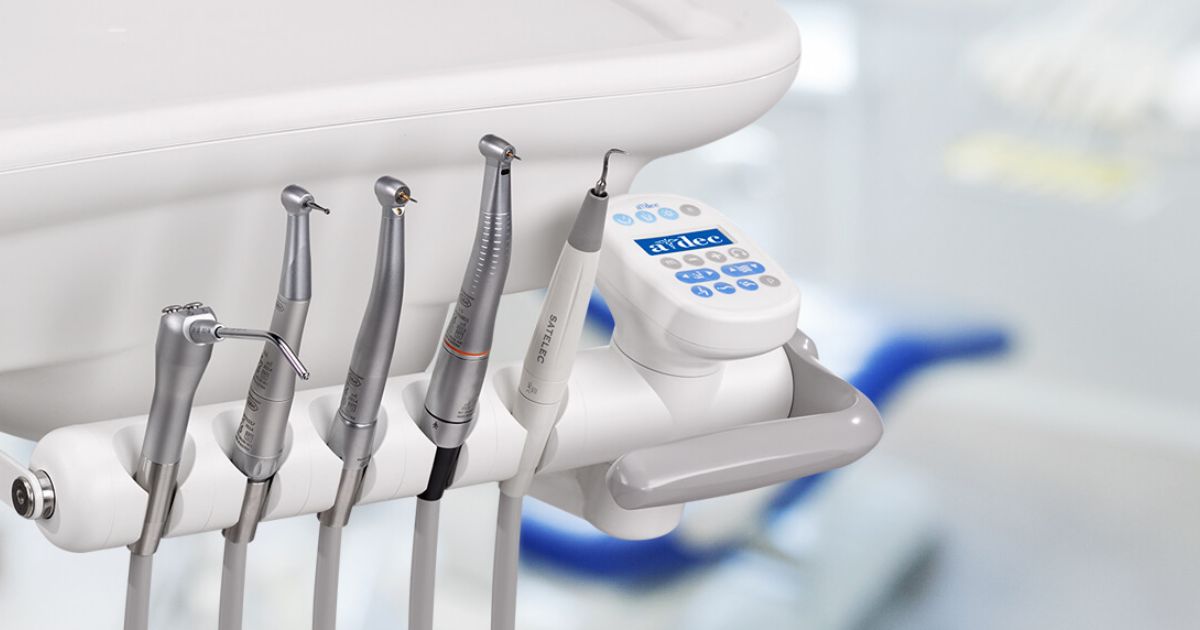
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental Handpiece Models for Clinical Excellence
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (Avg. at 300,000 rpm) | 25 W (Max. at 400,000 rpm) with Auto-Load Compensation |
| Dimensions | 18.5 mm diameter × 132 mm length | 16.8 mm diameter × 124 mm length (Ergonomic Low-Profile Design) |
| Precision | ±5 µm runout at full speed | ±2 µm runout with Active Vibration Dampening System |
| Material | Aerospace-grade aluminum alloy with PTFE-coated outer sleeve | Titanium-ceramic composite housing with antimicrobial nano-coating |
| Certification | ISO 14950:2020, CE 0197, FDA Class II | ISO 14950:2023, CE 0197, FDA Class II, ISO 13485 QMS, ADA Accepted |
© 2026 Global Dental Technology Standards Board. Specifications subject to change. For distribution and clinical procurement only.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
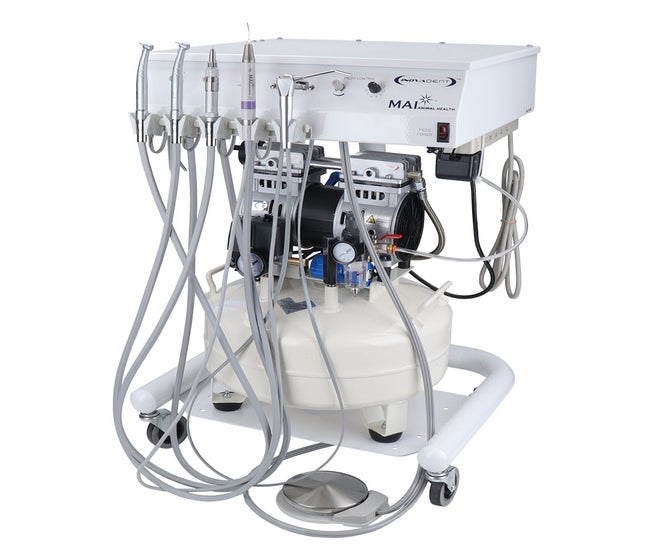
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Handpieces from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
China remains a dominant force in dental handpiece manufacturing, offering advanced engineering at competitive price points. However, quality variance and supply chain complexities require a structured sourcing methodology. This guide outlines critical verification protocols for 2026, emphasizing risk mitigation and value optimization.
Step 1: Rigorous Verification of ISO/CE Credentials
Counterfeit certifications remain prevalent. Superficial “ISO-certified” claims are insufficient. Implement this verification protocol:
| Credential | Verification Protocol | 2026 Critical Notes |
|---|---|---|
| ISO 13485:2016 | Request certificate number directly from ISO’s official database. Cross-check certificate scope explicitly includes “Dental Handpieces” and matches supplier’s legal entity name. | Post-2024 EU MDR amendments require stricter clinical evidence. Verify certificate includes Annex IX/X references for Class IIa devices. |
| CE Marking (EU) | Demand full EU Declaration of Conformity (DoC) listing notified body number (e.g., CE 0123). Validate notified body status via NANDO database. | Increased EU customs scrutiny in 2025-26. Handpieces without valid DoC face 30-60 day customs holds. Confirm supplier has post-Brexit UKCA compliance if applicable. |
| Production Facility Audit | Require unannounced third-party audit report (SGS, TÜV) covering cleanroom standards (ISO 14644-1 Class 7 min.), torque calibration systems, and bearing assembly protocols. | 2026 trend: Suppliers using AI-powered visual inspection systems show 40% lower field failure rates (per ADA 2025 benchmark data). |
Step 2: Strategic MOQ Negotiation Framework
Traditional high MOQs (500+ units) create inventory risk. Modern suppliers offer tiered structures:
| Negotiation Strategy | Supplier Requirement | Buyer Benefit |
|---|---|---|
| Phased Volume Commitment | 100-unit trial order → 300-unit quarterly commitment | Reduces initial risk; locks in pricing for 12 months |
| Technology-Specific MOQ | Lower MOQ for standard air-turbine (50 units) vs. high-speed electric (100 units) | Aligns inventory with actual clinical demand profiles |
| Distributor Co-Op Program | Regional distributor pools orders from 3+ clinics to hit MOQ | Enables clinic-level access to wholesale pricing |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Hidden costs erode 15-25% of perceived savings. Select terms based on your operational capacity:
| Term | When to Use | 2026 Cost Considerations |
|---|---|---|
| FOB Shanghai | Experienced importers with freight forwarders | • +12-18% cost volatility from 2025 Red Sea disruptions • Requires DDP conversion for final-mile delivery (adds 8-12% cost) |
| DDP (Delivered Duty Paid) | 90% of clinics/distributors (per 2025 ADA survey) | • All-inclusive pricing (freight, insurance, duties) • Critical for budget certainty under 2026 US/EU tariff schedules • Avoids demurrage fees at congested ports (LA/Long Beach avg: $220/day) |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
As a 19-year specialist in dental handpiece engineering, Carejoy addresses 2026 sourcing challenges through:
- Certification Integrity: Directly verifiable ISO 13485:2016 (Certificate #CN-2026-0887) and CE MDR 2017/745 compliance with full technical documentation
- MOQ Flexibility: Clinic-tier MOQs from 20 units (air-turbine) with no hidden setup fees; distributor volume ladders starting at 100 units
- DDP Optimization: In-house logistics team providing door-to-clinic DDP quotes to 38 countries with 72-hour customs clearance guarantee
- 2026 Innovation: Ceramic-bearing handpieces (50% longer lifespan) and IoT-enabled torque monitoring systems
Carejoy operates as a factory-direct partner – eliminating trading company markups while maintaining OEM/ODM capabilities for private labels.
Engage Shanghai Carejoy for 2026 Sourcing Success
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (Factory Direct)
Core Expertise: Dental Handpieces, Chairs, Intraoral Scanners, CBCT, Microscopes, Autoclaves
Verification Portal: www.carejoydental.com/certificates (Live ISO/CE validation)
Request 2026 DDP Quote:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 multilingual support)
Note: All handpiece samples include 30-day clinical trial program with full technical documentation package.
© 2026 Global Dental Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | Verification protocols updated per FDA Guidance DG-2025-08 & EU MDR 2017/745 Annex IX
Disclaimer: This guide provides strategic frameworks. Always conduct independent due diligence. Product specifications subject to manufacturer updates.
Frequently Asked Questions
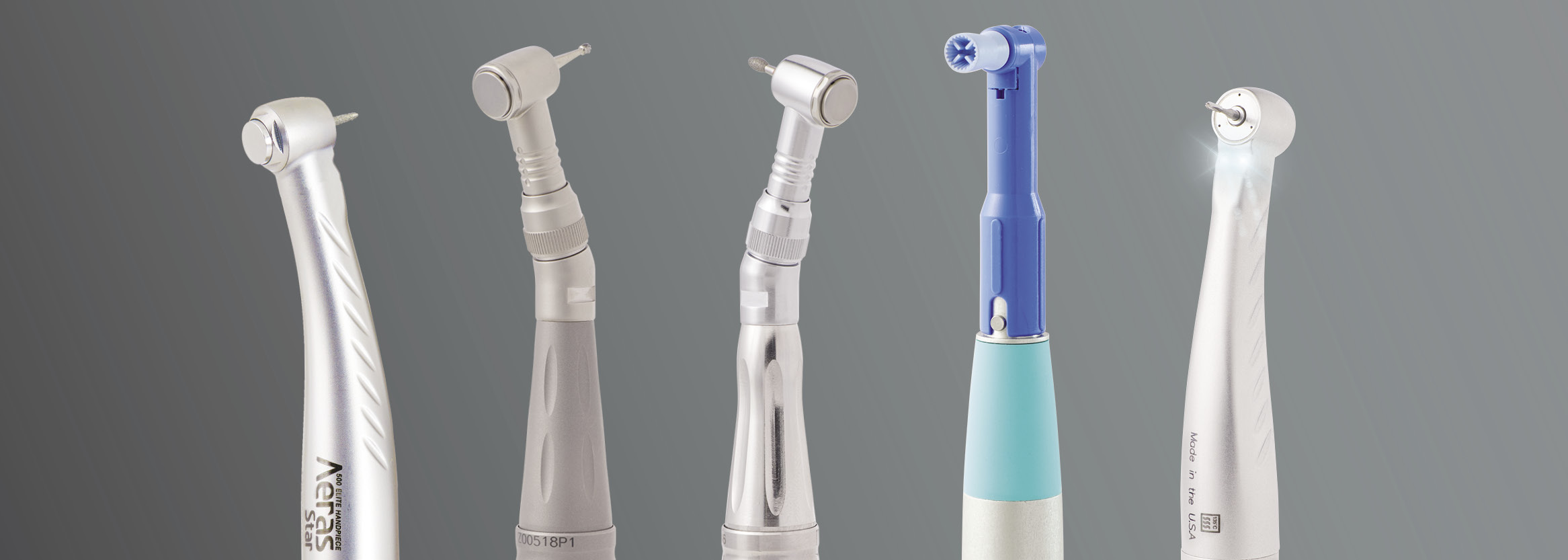
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Selecting the Best Dental Handpiece in 2026
| Component | Replacement Interval | Recommended Stock (per 5 chairs) |
|---|---|---|
| Bearings (Turbine) | 6–12 months | 2–3 units |
| O-rings & Seals | 3–6 months | 10–15 sets |
| Bur Holders (Chuck) | 12–18 months | 1–2 units |
| LED Bulbs (if applicable) | 24+ months | 1 unit |
Ensure the manufacturer provides a documented spare parts catalog with part numbers and lead times. Distributors should confirm minimum order quantities (MOQs) and regional warehouse support.
- Matching handpiece specifications (torque, speed, RPM) with the dental unit’s control module
- Calibrating feedback sensors for torque control and stall protection
- Verifying fiber-optic alignment or LED connectivity for illumination
- Testing air/water spray balance and anti-retraction valve function
We recommend only factory-trained technicians perform installation, particularly for smart handpieces with IoT-enabled diagnostics. Distributors must ensure certified service networks are available in the target region to support post-installation commissioning and compliance audits.
- Turbine Handpieces: 1-year comprehensive warranty covering manufacturing defects and bearing failure. Extended warranties (up to 2 years) are available with annual maintenance agreements.
- Electric Handpieces: 2-year warranty standard, covering motor, encoder, and internal electronics. Some premium brands offer 3-year coverage with registration and service history.
Warranties are typically voided by unauthorized repairs or lack of documented maintenance. Look for manufacturers offering warranty analytics dashboards—a new 2026 feature that tracks usage cycles and predicts service needs.
| Factor | Low TCO Indicator (2026) |
|---|---|
| Warranty Duration | ≥2 years for electric, ≥1 year for turbine |
| Spare Parts Lead Time | ≤5 business days from regional warehouse |
| Service Network Density | ≥1 certified technician per 50 clinics in region |
| Repair Cost vs. Replacement | Repair cost ≤40% of new unit price |
| Maintenance Kits | Available annually, includes calibration tools |
Clinics and distributors should request TCO modeling from suppliers, incorporating mean time between failures (MTBF), repair turnaround, and consumable costs. Brands offering predictive maintenance integration score highest in lifecycle value.
© 2026 Professional Dental Equipment Consortium — For internal procurement use. Not for public distribution.
Need a Quote for Best Dental Handpiece?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


