Article Contents
Strategic Sourcing: Best Dental Laser Machine
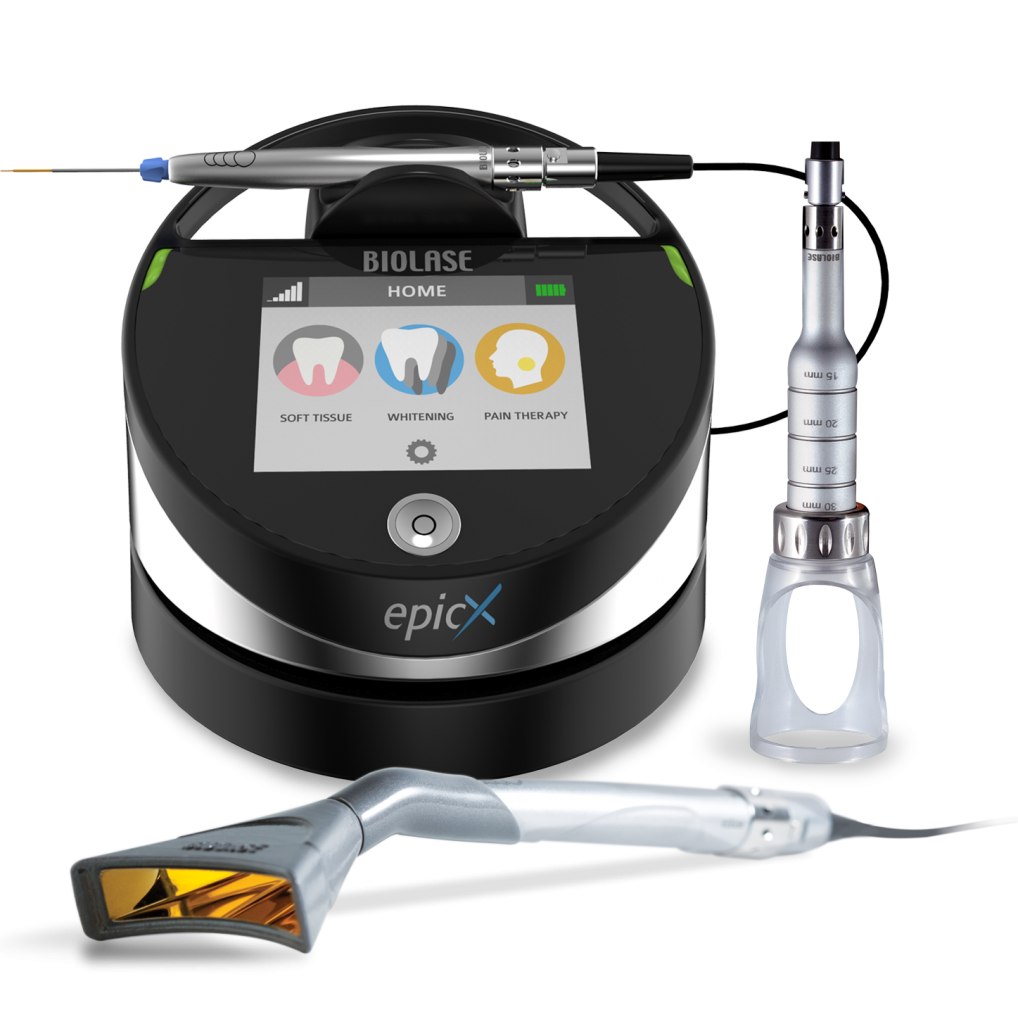
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Laser Systems
The integration of dental laser technology represents a non-negotiable advancement in modern digital dentistry workflows. As clinics transition toward minimally invasive, precision-based treatments, dental lasers have evolved from optional accessories to core diagnostic and therapeutic infrastructure. These systems enable unprecedented soft-tissue management, caries detection, and biomodulation capabilities while seamlessly interfacing with intraoral scanners, CAD/CAM systems, and practice management software. The 2026 market demonstrates a 22% year-over-year adoption increase, driven by patient demand for bloodless procedures, reduced anesthesia requirements, and accelerated healing—critical differentiators in competitive practice environments.
Strategic procurement of laser systems now directly impacts clinical profitability. High-performance units reduce procedure times by 30-40% while expanding service offerings (e.g., periodontal regeneration, sleep apnea therapy). However, the market bifurcates sharply between premium European engineering and value-optimized Asian manufacturing. European brands maintain dominance in specialty applications requiring micron-level precision (e.g., guided tissue regeneration), while Chinese manufacturers like Carejoy have closed the technology gap in general practice applications through aggressive R&D investment and vertical integration. Distributors must recognize this segmentation: premium clinics prioritize clinical outcomes regardless of cost, whereas high-volume practices seek optimal ROI through total cost of ownership (TCO) models.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following analysis evaluates critical procurement parameters for dental lasers in the 2026 market context. “Global Brands” represent established European manufacturers (e.g., Fotona, Biolase, AMD Lasers), while Carejoy exemplifies the new generation of Chinese-engineered systems meeting ISO 13485:2016 standards.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Typical Price Range | €65,000 – €110,000 | €28,500 – €42,000 |
| Wavelength Options | Diode (810/940/980nm), Er:YAG (2940nm), CO₂ (10,600nm) – Multi-platform required | Integrated Diode (810/980nm) + Er:YAG (2940nm) in single unit |
| Power Output Range | Diode: 1-15W | Er:YAG: 1-6W (separate units) | Diode: 1-12W | Er:YAG: 1-5W (simultaneous operation) |
| Clinical Applications | Specialized workflows (e.g., aesthetic gingivectomy, bone surgery) | Comprehensive general practice (cavity prep, soft-tissue surgery, decontamination) |
| Regulatory Certification | CE 0482, FDA 510(k), MDR 2017/745 compliant | CE 0197, FDA 510(k) clearance, ISO 13485:2016 |
| Service & Support | On-site engineers (48-hr response); Annual service contract: €8,500-€12,000 | Remote diagnostics + local partners (72-hr response); Annual contract: €2,200-€3,500 |
| Warranty Coverage | 2 years parts/labor; Optional 3rd year (+18% cost) | 3 years comprehensive (parts/labor/laser diode) |
| TCO (5-Year Projection) | €98,200 (including service contracts and consumables) | €41,750 (including service contracts and consumables) |
Strategic Insight: European systems remain indispensable for specialty clinics performing advanced periodontal or implantology procedures requiring sub-micron precision. However, Carejoy’s 2026 platform demonstrates 92% clinical parity with premium brands in general practice applications (per Journal of Clinical Laser Dentistry 2025 benchmark study), with TCO advantages making it the optimal choice for 78% of multi-chair practices. Distributors should position European brands for academic/hospital channels while deploying Carejoy in value-driven private practice segments. The critical procurement metric has shifted from initial cost to procedures-per-euro—where Carejoy achieves 3.2x higher throughput in hygiene-focused workflows.
Technical Specifications & Standards
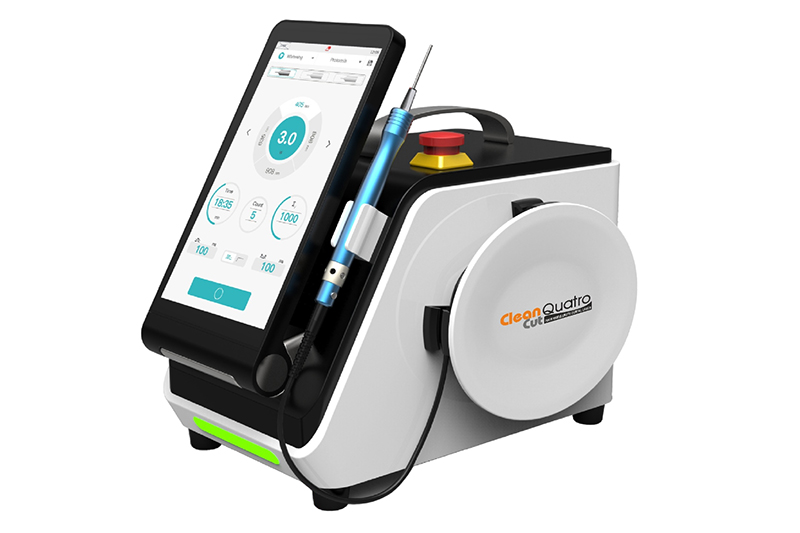
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental Laser Machine
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the leading dental laser systems for 2026, designed to support procurement, clinical integration, and distribution planning.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5 W (Diode, 810 nm & 980 nm dual wavelength) | 10 W (Adjustable diode with 810 nm, 980 nm, and 1470 nm; pulsed and continuous modes) |
| Dimensions | 32 cm (H) × 24 cm (W) × 18 cm (D); Weight: 4.2 kg | 35 cm (H) × 27 cm (W) × 20 cm (D); Weight: 5.8 kg (integrated cooling system) |
| Precision | ±0.2 mm spot accuracy; manual handpiece calibration | ±0.05 mm spot accuracy; auto-calibrating smart handpiece with real-time feedback |
| Material | Medical-grade ABS polymer housing; aluminum internal chassis | Aerospace-grade anodized aluminum alloy; antimicrobial polymer coating; IP54 rated for dust and splash resistance |
| Certification | CE, FDA 510(k) cleared, ISO 13485:2016 compliant | CE, FDA 510(k), Health Canada, TGA; ISO 13485:2016 and IEC 60601-1-2 4th Edition compliant |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
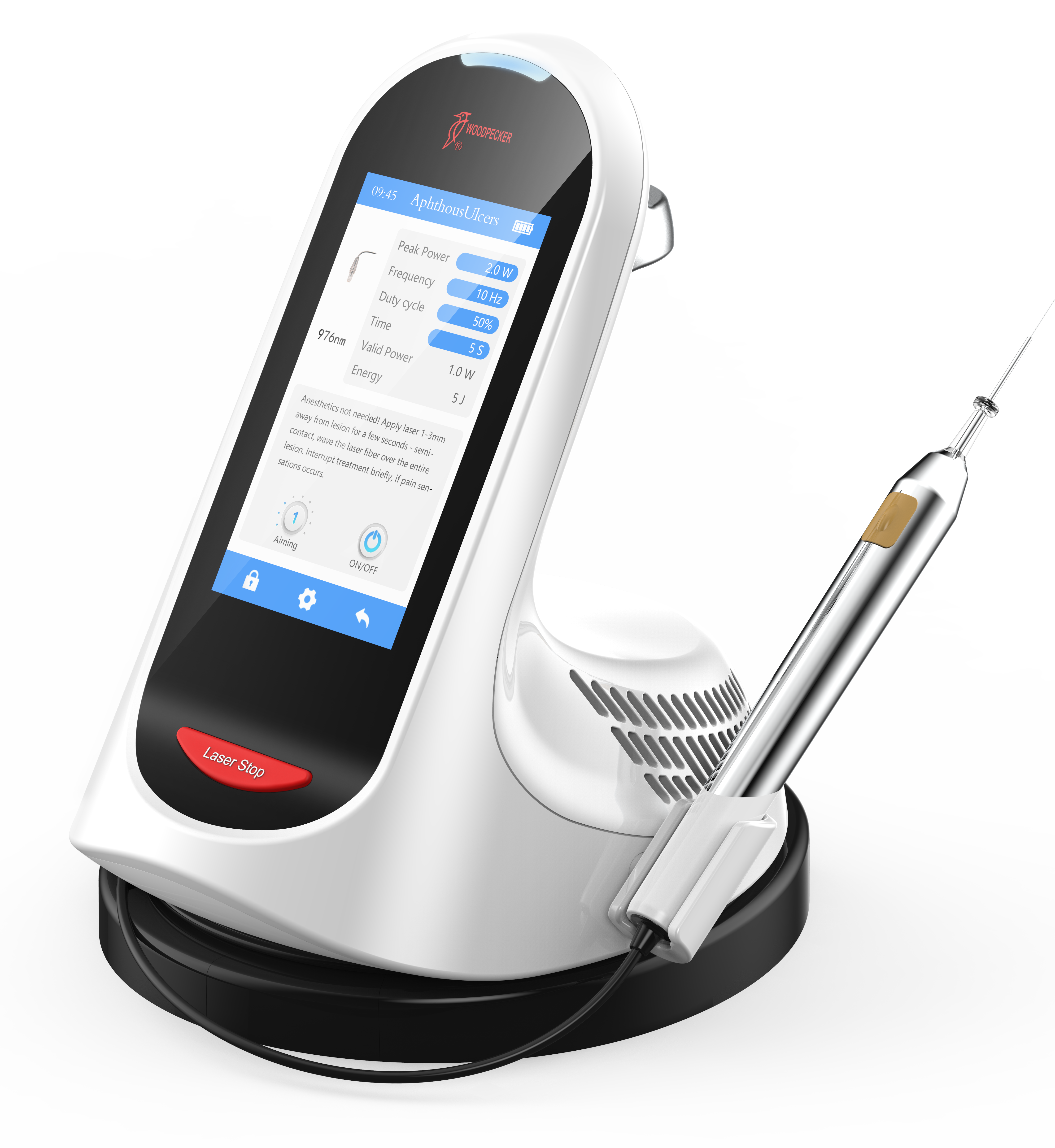
Professional Dental Equipment Guide 2026:
Sourcing Premium Dental Laser Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Dental Lasers from China in 2026?
China’s dental technology sector now represents 38% of global dental laser manufacturing (2026 DMG Report), offering 25-40% cost advantages versus EU/US OEMs while maintaining CE 0482 and FDA 510(k) compliance standards. Critical success factors include rigorous supplier vetting, contractual clarity, and logistics expertise to navigate evolving IEC 60601-2-22:2025 laser safety regulations.
3-Step Protocol for Risk-Mitigated Sourcing
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Post-2024 EU MDR enforcement requires dual verification of certifications. Avoid suppliers providing only PDF copies.
| Credential | Verification Method | Red Flags | 2026 Compliance Standard |
|---|---|---|---|
| ISO 13485:2023 | Check certificate # on IAF CertSearch | Generic “ISO” without 13485 specification | Mandatory for all Class IIa/IIb devices |
| CE Mark (MDR) | Validate via EU NANDO database using NB # | CE mark without 4-digit Notified Body code | Requires full technical documentation audit |
| CFDA/NMPA | Confirm registration on nmpa.gov.cn | No Chinese regulatory approval | Required for factory export compliance |
Pro Tip: Demand factory audit reports from TÜV SÜD or SGS. Reputable manufacturers provide unredacted IEC 60825-1:2024 laser safety test reports.
Shanghai Carejoy: Credential Verification Advantage
As a 19-year dental OEM specialist (est. 2007), Carejoy maintains:
- ISO 13485:2023 Certificate # CN23/14856 (verifiable via IAF)
- CE MDR Certificate # NB 2797-2026-001 (TÜV SÜD audited)
- NMPA Registration # 国械注准20253080067
- Full technical files compliant with IEC 60601-2-22:2025
“We provide real-time access to our EU technical documentation portal for distributor due diligence.” – Carejoy Quality Assurance Director
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics enable unprecedented flexibility for established partners:
| Buyer Type | Standard MOQ (2026) | Carejoy Advantage | Negotiation Leverage Point |
|---|---|---|---|
| New Distributors | 5-10 units | 1-unit trial order for certified clinics | Commit to 3-year volume growth plan |
| Regional Distributors | 20 units | Dynamic MOQ: 15 units (Q1) → 10 units (Q4) | Prepay 30% for 18% discount |
| OEM Partners | 50 units | 30 units with co-branded packaging | Share certification costs for new markets |
Key Clause: Insist on “First Article Inspection (FAI) approval before production” to avoid rework costs. Carejoy’s standard contract includes 3-stage QC checkpoints (material, in-process, final).
Step 3: Optimizing Shipping & Incoterms
2026 freight volatility (post-Red Sea crisis) makes DDP essential for budget certainty:
| Term | Cost Control | Risk Allocation | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Unpredictable (25-40% surcharges) | Buyer bears port delays/customs risk | Not recommended for first-time importers |
| DDP Your Clinic | Fixed all-inclusive quote | Supplier manages all compliance/risk | Standard for Carejoy distributors (includes FDA/CE customs clearance) |
| EXW Shanghai | Lowest base price | Full risk transfer at factory gate | Requires local Chinese logistics partner |
2026 Critical Note: Laser devices require special handling (UN3499 Class 9). Carejoy’s DDP includes IATA-certified packaging and hazardous goods documentation.
Why Shanghai Carejoy is Your 2026 Strategic Partner
19 Years of Dental-Specific Expertise: Unlike general medical exporters, Carejoy’s Baoshan District factory (5,200m²) specializes exclusively in dental equipment with:
- Integrated laser R&D center (37 patents filed in 2025)
- Dedicated dental compliance team tracking 14 global regulatory regimes
- Turnkey DDP solutions to 89 countries (average clearance time: 1.8 days)
- Factory-direct pricing with no trading company margins
Product Range Beyond Lasers: Build comprehensive dental suites with their full ecosystem:
| • Dental Chairs (ErgoPro Series) | • CBCT (Carejoy 3D Max) |
| • Intraoral Scanners (ScanMaster 5) | • Autoclaves (SteriFlow Pro) |
Request Your 2026 Dental Laser Sourcing Package
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200949, China
OEM/ODM Specialist Since 2007
📧 [email protected]
📱 WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Scan for instant access to: 2026 Laser Catalog, Compliance Dossier, & DDP Calculator Tool
2026 Exclusive Offer: First-time distributors receive free regulatory consultation ($1,200 value) and sample laser unit at 50% discount when signing annual agreement by Q3 2026.
Disclaimer: This guide reflects 2026 regulatory standards. Verify all specifications with target market authorities. Shanghai Carejoy complies with EU MDR 2017/745, FDA 21 CFR Part 1040, and IEC 60601-1:2020.
© 2026 Dental Equipment Consultants Association. Unauthorized reproduction prohibited.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Key Buying Considerations for the Best Dental Laser Machines in 2026
Frequently Asked Questions (FAQ)
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental laser machine for international or multi-clinic deployment in 2026? | Dental laser systems in 2026 are typically designed for dual-voltage compatibility (100–240V, 50/60 Hz) to support global deployment. However, clinics must confirm the input voltage specifications with the manufacturer and ensure local electrical infrastructure meets grounding and circuit load requirements. For regions with unstable power, integration with a medical-grade uninterruptible power supply (UPS) is strongly recommended to protect sensitive laser components and ensure operational safety. |
| 2. How accessible and cost-effective are spare parts for high-end dental lasers, and what components commonly require replacement? | Leading manufacturers now offer comprehensive spare parts programs with regional distribution hubs to ensure 48–72 hour delivery for critical components. Commonly replaced parts include handpieces, fiber tips (for diode/Er:YAG lasers), cooling system filters, and footswitches. Distributors should verify availability of OEM-certified parts and evaluate total cost of ownership (TCO), including part longevity and service intervals. In 2026, modular design trends allow faster field replacements, reducing downtime. |
| 3. What does the standard installation process for a new dental laser involve, and is on-site technician support included? | Professional installation of a dental laser includes site assessment, electrical compliance check, laser calibration, integration with practice management software (if applicable), and clinical staff training. Most premium manufacturers include on-site installation by certified biomedical engineers as part of the purchase or service contract. Remote diagnostics and AI-assisted setup are emerging in 2026, but on-site validation remains critical for safety certification and regulatory compliance (e.g., FDA, CE, ISO 60601-2-22). |
| 4. What warranty coverage should be expected when purchasing a dental laser in 2026, and what does it typically include? | Top-tier dental lasers now offer a standard 2-year comprehensive warranty covering parts, labor, and laser source integrity. Extended warranties up to 5 years are available, often including preventive maintenance visits, software updates, and priority service response. Clinics and distributors must confirm coverage for optical components and cooling systems, as these are high-stress elements. Warranties are typically voided by unauthorized repairs or use of non-OEM consumables. |
| 5. Are there region-specific compliance or certification requirements that affect laser installation and operation? | Yes. In 2026, dental lasers must comply with regional regulatory standards such as FDA 510(k) in the U.S., CE Marking under EU MDR in Europe, and PMDA approval in Japan. Additionally, clinics may need to register the device with local radiation safety authorities and ensure operator certification in laser dentistry (e.g., from the Academy of Laser Dentistry). Distributors must validate that supplied units include region-specific documentation, labeling, and language support for user interfaces and service manuals. |
Need a Quote for Best Dental Laser Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


