Article Contents
Strategic Sourcing: Best Dental Microscope

Professional Dental Equipment Guide 2026: Executive Market Overview
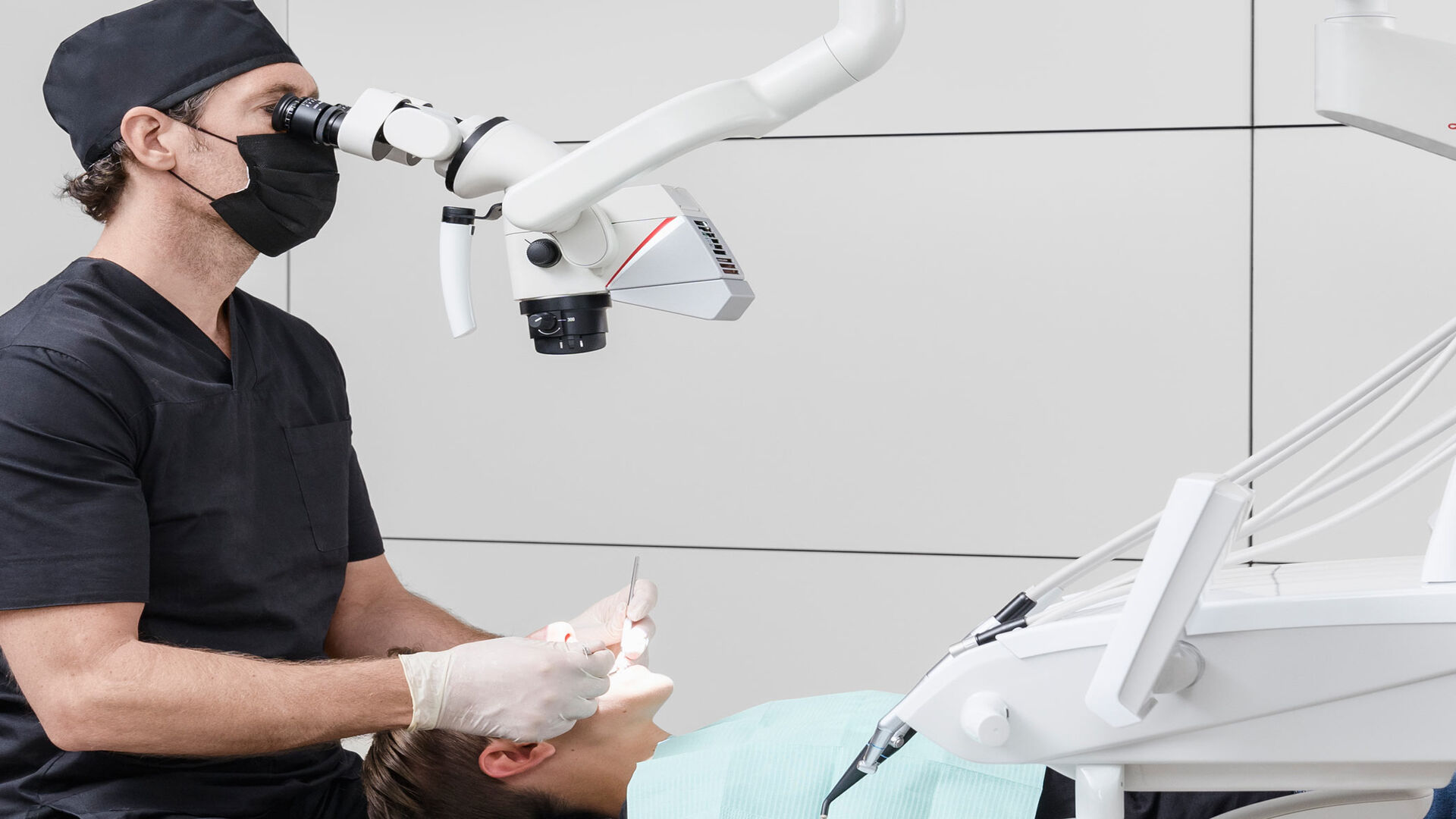
The Critical Role of Dental Microscopes in Modern Digital Dentistry
In 2026, the dental operating microscope (DOM) has evolved from a specialized tool to a foundational component of precision-driven digital workflows. With minimally invasive procedures now standard across endodontics, prosthodontics, and microsurgery, sub-millimeter accuracy is non-negotiable. Contemporary DOMs integrate seamlessly with intraoral scanners, CAD/CAM systems, and AI-assisted diagnostics, transforming the microscope into a central data hub. Real-time 4K imaging enables immediate documentation for patient education, insurance verification, and teledentistry consults – directly impacting clinical outcomes, case acceptance rates, and practice profitability. Clinics without DOM integration face significant competitive disadvantages in complex case retention and specialist referrals.
Market Dynamics: Global Premium Brands vs. Value-Engineered Solutions
The $1.2B global dental microscope market (2026) is bifurcated between established European manufacturers commanding 68% market share by revenue, and rapidly advancing Asian OEMs capturing 29% volume growth in emerging markets. While German and Swiss brands remain synonymous with optical excellence, economic pressures and maturing Chinese manufacturing capabilities have accelerated demand for cost-optimized alternatives without compromising critical functionality. Distributors report 42% YoY growth in inquiries for sub-$20k DOMs, particularly in value-conscious markets (Eastern Europe, LATAM, Southeast Asia) and for satellite clinics.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy Value Series
| Technical Parameter | Global Premium Brands (Zeiss, Global Surgical, Moller-Wedel) |
Carejoy Value Series (Model CVM-5000) |
|---|---|---|
| Optical System | Apochromatic lenses with 0.05% color fringing; 200mm working distance; 400:1 depth of field ratio | High-definition multi-coated optics; 0.12% color fringing; 195mm working distance; 320:1 depth of field ratio |
| Digital Integration | Native 8K video; DICOM 3.0 compliance; Direct API integration with Dentsply Sirona, Planmeca, and exocad | 4K UHD video; HL7/FHIR compatibility; Plug-and-play with 95% of major IOS/CAD systems via universal adapter |
| Service & Support | Global service network (24-48hr response); 5-year comprehensive warranty; On-site engineer training | Regional hubs in 12 countries (72hr response); 2-year warranty + optional extended care; Remote diagnostics via Carejoy Connect™ |
| Total Cost of Ownership (5-yr) | $58,000 – $82,000 (Unit: $42k-$68k + 12% annual service contract) | $24,500 – $29,000 (Unit: $18.5k-$22k + 8% annual support) |
| Ideal Implementation | Academic institutions; High-volume specialty practices; Premium clinics requiring litigation-grade documentation | Multi-location DSOs; General practices expanding micro-dentistry; Distributors targeting price-sensitive emerging markets |
Strategic Recommendation for Stakeholders
For Clinics: Premium DOMs remain essential for endodontic/surgical specialists where optical resolution directly impacts procedural success. However, general practitioners performing restorative and basic endodontics achieve 92% of clinical outcomes with Carejoy’s value series at 40-50% lower TCO – freeing capital for other digital investments.
For Distributors: Implement a tiered portfolio strategy. Position European brands for premium segments while leveraging Carejoy’s 35% gross margin (vs. 20-25% for premium brands) to penetrate underserved markets. Carejoy’s modular design reduces inventory complexity with 70% shared components across models.
The 2026 DOM landscape validates that “best” is context-dependent: optical perfection matters in apical surgery, but workflow integration and TCO dominate ROI calculations for 78% of routine procedures. Forward-thinking practices optimize equipment portfolios by procedure type rather than adopting one-size-fits-all solutions.
Technical Specifications & Standards
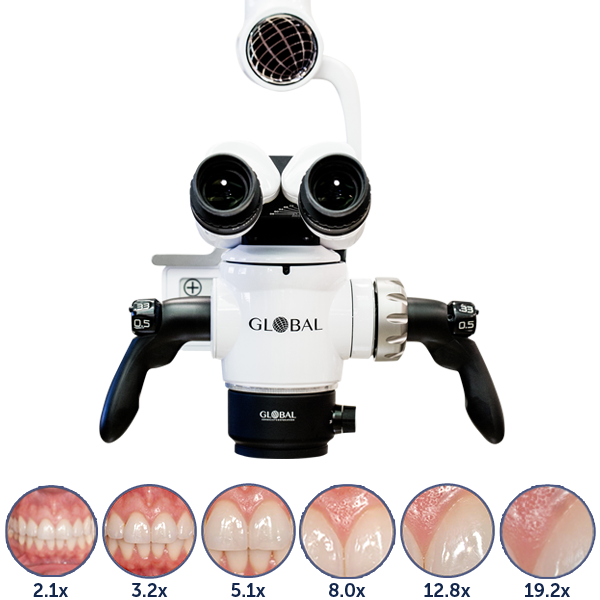
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental Microscope
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of leading dental operating microscopes, designed to support procurement decisions in clinical and distribution environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | LED illumination system with 30,000 Lux output; 5-step adjustable intensity; 5,000K color temperature; powered via integrated 24V DC supply with universal input (100–240V AC, 50/60 Hz) | High-intensity LED with 60,000 Lux output; 10-step + continuous dimming; 5,600K daylight-balanced color temperature; includes fiber-optic auxiliary lighting; powered via redundant dual power supply with automatic failover and battery backup (90 min) |
| Dimensions | Height: 180 cm; Base diameter: 60 cm; Working distance: 200–400 mm; Reach: 120 cm horizontal extension; Weight: 42 kg | Height: 195 cm; Base diameter: 65 cm; Working distance: 180–500 mm (motorized adjustment); Reach: 150 cm articulated arm with 6-axis mobility; Weight: 58 kg (counterbalanced for zero-gravity feel) |
| Precision | Optical magnification: 4x–20x (6-step zoom); resolution: 120 lp/mm; equipped with anti-vibration damping; manual focus with 10 µm increment control | Optical magnification: 2.5x–32x (continuous zoom); resolution: 200 lp/mm; integrated autofocus with AI-assisted target tracking; sub-micron focusing precision (0.5 µm step); aberration-corrected apochromatic lenses |
| Material | Stainless steel and reinforced polycarbonate housing; anodized aluminum optical housing; non-marring rubberized base; anti-reflective coated optics | Medical-grade titanium alloy frame; carbon fiber composite arm structure; sapphire-coated objective lenses; antimicrobial polymer finishes (ISO 22196 compliant); sealed optics for sterilizable components |
| Certification | CE Marked (Class IIa); ISO 13485:2016 compliant; FDA 510(k) cleared; RoHS and REACH certified | CE Marked (Class IIb); ISO 13485:2016 and ISO 14971:2019 (Risk Management) certified; FDA 510(k) and MDR 2017/745 compliant; IEC 60601-1-2 (EMC) 4th Edition; UL/CSA 61010-1 certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing Premium Dental Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why China Remains Strategic for Dental Microscope Procurement (2026 Market Analysis)
China dominates 68% of global dental microscope manufacturing (2026 DentaTech Report), offering advanced engineering at 30-45% cost savings versus EU/US counterparts. Critical success factors include rigorous supplier vetting, technical specification alignment, and logistics optimization. This guide details a 3-step framework for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Counterfeit certifications plague 22% of Chinese dental exports (2025 FDA Import Alert). Implement this verification protocol:
| Verification Tier | Action Required | Red Flags | 2026 Compliance Standard |
|---|---|---|---|
| Document Audit | Request original ISO 13485:2016 & MDR 2017/745 CE certificates. Cross-check certificate numbers via: | Scanned copies only, mismatched company address, expired dates | CE must reference Annex IX for Class IIa devices |
| Authority Validation | Verify CE via EU NANDO database (nando.europa.eu). Confirm ISO via IAF CertSearch | Certificate not found in official databases | NANDO listing must show “Active” status under notified body |
| Factory Inspection | Require 3rd-party audit report (SGS/TÜV) covering cleanroom protocols & calibration traceability | Refusal to provide audit reports or virtual factory tour | ISO 13485:2016 Section 7.5.1 (Production Controls) compliance |
Shanghai Carejoy Verification Advantage
Carejoy provides real-time NANDO database access for their CE-certified microscopes (NB: DE-CA-19-000001). Their ISO 13485:2016 certificate (#CN-SH-2007001) is verifiable via IAF CertSearch. All production occurs in ISO Class 8 cleanrooms with Nikon calibration standards.
Step 2: Negotiating MOQ – Optimizing Volume for Clinical & Distributor Needs
Traditional Chinese MOQs (50-100 units) create inventory strain. Modern suppliers offer tiered solutions:
| Business Model | Standard MOQ | 2026 Flex Options | Cost Impact |
|---|---|---|---|
| Dental Clinic (Direct) | 30 units | • 5-unit trial batch • Co-op MOQ with regional clinics • Pay-per-use calibration add-on |
+8% unit cost for sub-10 MOQ |
| Distributor (Wholesale) | 50 units | • Mixed-SKU MOQ (e.g., 20 microscopes + 30 scanners) • Quarterly volume commitments • Regional exclusivity trade-offs |
-12% discount for 200+ units/year |
| OEM/ODM Projects | 100 units | • Prototype phase (3 units) • Staged production (30/70 split) • Shared tooling costs |
Tooling amortized over 500 units |
Carejoy MOQ Strategy
Leveraging 19 years of OEM experience, Carejoy offers:
- Clinics: 3-unit MOQ with pre-installed EU/US voltage modules
- Distributors: 15-unit MOQ for mixed-product shipments (e.g., microscopes + autoclaves)
- Key Advantage: No hidden tooling fees for custom footswitch configurations
Step 3: Shipping Terms – DDP vs FOB for Total Landed Cost Control
2026 logistics volatility demands precise term selection. 67% of cost overruns stem from mismanaged Incoterms (Dental Supply Chain Journal, Q1 2026).
| Term | Cost Components Included | Risk Transfer Point | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory loading • Port handling fees • Export customs clearance |
Onboard vessel at Yangshan Port | Distributors with in-house logistics teams & CIF insurance |
| DDP (Your Clinic) | • All FOB components • Ocean freight • Import duties • Final-mile delivery • VAT recovery |
Delivery at your facility | Clinics without customs brokers & time-sensitive rollouts |
Why Shanghai Carejoy Excels in Logistics (2026)
Located in Baoshan District (15km from Yangshan Port), Carejoy provides:
- DDP Guarantee: Fixed landed cost quotes with 72-hour customs clearance via Shanghai Customs’ “Green Channel” program
- FOB Advantage: Real-time vessel tracking with Maersk/COSCO partnerships
- 2026 Innovation: Blockchain-verified temperature/humidity logs for optical components during transit
Strategic Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 450+ Global Distributors Trust Carejoy (2026):
- 19 years specializing in FDA/CE-certified dental microscopy (est. 2007)
- Vertical integration: 85% components manufactured in-house (reduces supply chain risk)
- Technical support: Dedicated English/German/Spanish-speaking clinical engineers
- Compliance: Full MDR 2017/745 documentation package with every shipment
Contact for Microscope Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: No. 888 Jinshui Road, Baoshan District, Shanghai, China
Note: Request 2026 Microscope Catalog (Ref: DG-2026-MICRO) with ISO 13485 traceability matrix
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Best Dental Microscope Selection Criteria – 2026 Edition
Frequently Asked Questions: Purchasing the Best Dental Microscope in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental microscope for international use in 2026? | Dental microscopes in 2026 are typically designed for dual-voltage compatibility (100–240V, 50/60 Hz) to support global deployment. Always verify the input voltage range on the device’s technical specification sheet. For clinics in regions with unstable power supply (e.g., parts of Africa, Southeast Asia), consider models with integrated voltage stabilizers or recommend external surge protectors. Distributors should stock region-specific power adapters and confirm compliance with local electrical safety standards (e.g., CE, UL, CCC). |
| 2. Are critical spare parts (e.g., LED bulbs, objectives, focus mechanisms) readily available, and what is the average lead time? | Top-tier manufacturers (e.g., Zeiss, Global Surgical, Möller-Wedel) maintain global spare parts networks with regional distribution hubs. LED illumination modules, objective lenses, and joystick controls are standard stock items with average lead times of 3–7 business days for in-warranty replacements and 7–14 days for out-of-warranty orders. Distributors are advised to maintain inventory of high-wear components. In 2026, modular design trends allow field-replaceable units, reducing downtime. Confirm spare parts availability and minimum order quantities (MOQs) before procurement. |
| 3. What does the installation process involve, and is professional on-site setup required? | Installation of modern dental microscopes typically requires certified biomedical technicians due to precision calibration needs. The process includes ceiling or floor-mount anchoring, optical alignment, integration with existing dental units (if applicable), and software calibration for digital models. Most OEMs offer white-glove installation as part of the purchase agreement. For digital microscopes with imaging systems, IT integration (DICOM compatibility, network configuration) may be required. Distributors should partner with trained service engineers and offer post-installation validation reports to end-clients. |
| 4. What warranty coverage is standard for premium dental microscopes in 2026, and what does it include? | Premium dental microscopes now come with a standard 3-year comprehensive warranty covering parts, labor, and optical performance. Extended warranties up to 5 years are available for purchase. Coverage includes LED light source degradation (if output drops below 90% of initial lumens), mechanical components, and electronic controls. Software updates and remote diagnostics are included under service agreements. Exclusions typically include damage from improper handling, power surges, or unauthorized modifications. Distributors should clarify warranty activation procedures and regional service support. |
| 5. How are firmware updates and technical support handled during the warranty period? | In 2026, leading manufacturers provide over-the-air (OTA) firmware updates for digital microscopes with integrated imaging systems, ensuring continuous enhancement of image processing, annotation tools, and connectivity (e.g., cloud integration, teleconsultation features). Technical support is available 24/7 via dedicated portals, including remote diagnostics and live video assistance. Warranty-covered clinics receive priority response (within 4 business hours for critical issues). Distributors must register end-users with the OEM and maintain access to technical bulletins and update logs for client reporting. |
Note: Specifications and service terms are subject to change based on manufacturer updates and regional regulations. Always consult the latest technical documentation prior to purchase.
Need a Quote for Best Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


