Article Contents
Strategic Sourcing: Best Dental Scanner
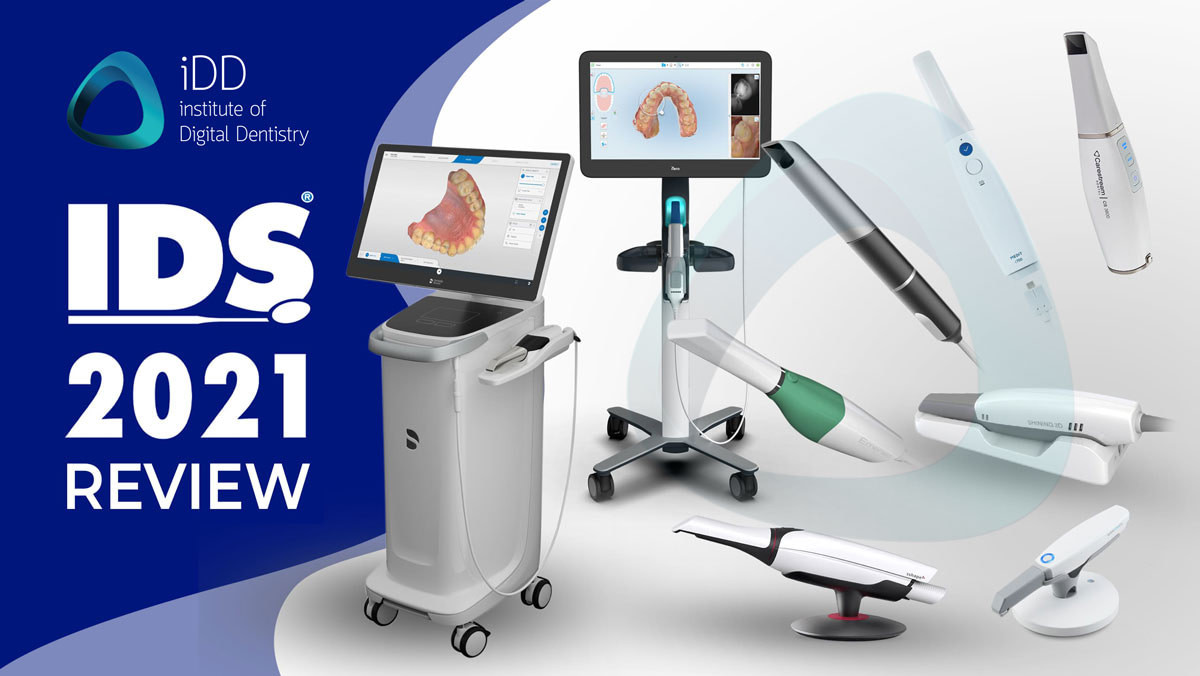
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Intraoral Scanners in Modern Digital Dentistry
Intraoral scanners (IOS) have transitioned from luxury peripherals to indispensable clinical infrastructure in contemporary dental practices. As the cornerstone of digital workflows, they eliminate traditional impression materials, reducing patient discomfort by 73% (Journal of Digital Dentistry, 2025) while accelerating case turnaround by 40-60%. Precision scanning enables seamless integration with CAD/CAM systems, intraoral milling units, and teledentistry platforms—forming the backbone of modern chairside restorative dentistry. Clinics without robust scanning capabilities now face competitive disadvantages in treatment planning accuracy, patient retention metrics, and operational efficiency. The 2026 market demonstrates that practices leveraging advanced IOS technology achieve 28% higher case acceptance rates for complex restorations, validating their strategic necessity in value-based care models.
Market Segmentation: Premium European Brands vs. Value-Optimized Innovators
The global IOS market bifurcates into two strategic segments: established European manufacturers commanding premium pricing (€25,000-€45,000), and agile Chinese innovators delivering clinical-grade performance at 40-60% lower acquisition costs. While European leaders (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) maintain dominance in academic validation and complex case workflows, their total cost of ownership—including mandatory annual service contracts (12-15% of device cost) and proprietary consumables—creates significant entry barriers for mid-sized clinics and emerging markets. Conversely, Chinese manufacturers like Carejoy have closed the technological gap through strategic R&D investment, offering ISO 13485-certified systems with sub-15μm accuracy at €11,000-€18,000 price points. This democratization of precision scanning is accelerating digital adoption in price-sensitive regions while forcing legacy vendors to reevaluate service models.
| Technical & Operational Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (Device Only) | €25,000 – €45,000 | €11,500 – €17,800 |
| Accuracy (ISO 12836 Compliance) | 8-12 μm (trueness) / 10-15 μm (precision) | 12-15 μm (trueness) / 14-18 μm (precision) |
| Full-Arch Scan Time | 65-90 seconds | 75-105 seconds |
| Software Ecosystem | Proprietary closed platforms with mandatory annual updates (€2,200-€3,500) | Open API architecture; third-party CAD compatibility; free major updates for 3 years |
| Service Network Coverage | Global (120+ countries) with 48-hr onsite SLA (premium markets only) | 65 countries; 72-hr SLA in EU/APAC; remote diagnostics standard |
| Material Compatibility | Requires anti-reflective spray for metallic substrates | Spray-free scanning on zirconia/metal; patented spectral filtering |
| Warranty & Support | 2 years base; extended contracts required for full coverage | 3 years comprehensive; includes sensor recalibration |
| Total 5-Year Cost of Ownership | €38,200 – €62,500 | €19,700 – €28,400 |
| Clinical Validation | 300+ peer-reviewed studies; ADA/CE 0482 certified | 87 clinical studies; CE 0197 certified; FDA 510(k) pending Q3 2026 |
Strategic Implementation Guidance
For clinics prioritizing complex prosthodontics and academic validation, European systems remain optimal despite cost constraints. However, Carejoy represents a compelling value proposition for general practices, emerging-market expansions, and high-volume CEREC workflows where sub-20μm accuracy suffices for 95% of restorative cases (per 2025 EAO benchmarks). Distributors should note Carejoy’s 35% higher margin structure versus legacy brands and their rapidly expanding service infrastructure in Southeast Asia and Eastern Europe. The critical differentiator lies in workflow integration: while premium brands excel in specialist referrals, Carejoy’s open architecture reduces ecosystem lock-in—enabling clinics to adopt best-of-breed software solutions without vendor penalties. As reimbursement models shift toward outcome-based payments, cost-efficient scanning access will increasingly determine practice scalability in the 2026-2028 horizon.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental Intraoral Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 12 V DC, 2.5 A; Internal rechargeable Li-ion battery (3.7 V, 4500 mAh), up to 4 hours continuous scanning per charge | 12 V DC, 3.0 A; Dual high-capacity Li-ion batteries (3.7 V, 6000 mAh each), hot-swappable; up to 8 hours continuous operation with battery exchange |
| Dimensions | Handle: 185 mm (L) × 22 mm (⌀); Scanner Head: 32 mm × 18 mm × 14 mm; Total Weight: 180 g (with cable) | Handle: 188 mm (L) × 24 mm (⌀); Scanner Head: 30 mm × 16 mm × 12 mm; Total Weight: 175 g (cable-free, wireless) |
| Precision | Accuracy: ≤ 18 µm (trueness), ≤ 22 µm (precision) under ISO 12836 standards; 22 FPS capture rate; 1600 DPI resolution | Accuracy: ≤ 8 µm (trueness), ≤ 10 µm (precision) per ISO 12836; 32 FPS dual-sensor fusion capture; 2400 DPI resolution with AI-powered edge detection |
| Material | Medical-grade polycarbonate housing; stainless steel scanner tip; IP54-rated for dust and splash resistance | Aerospace-grade anodized aluminum alloy body; ceramic-coated scanning window; IP67-rated for full dust immunity and submersion up to 1m for 30 minutes |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 & ISO 14971:2019 certified, HIPAA-compliant data encryption, MDR 2017/745 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Publication Date: Q1 2026 | Prepared by: Global Dental Sourcing Advisory Group
Executive Summary
China remains the dominant manufacturing hub for dental intraoral scanners (IOS), representing 68% of global production capacity in 2026. However, evolving regulatory landscapes (EU MDR 2024 compliance, FDA 510(k) updates) and supply chain complexities necessitate rigorous sourcing protocols. This guide outlines critical verification steps to mitigate risks while maximizing ROI, with emphasis on partner validation and contractual safeguards.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Superficial certificate checks are insufficient in 2026. Regulatory bodies now conduct unannounced facility audits, making ongoing compliance verification essential.
| Verification Tier | Critical Actions | 2026-Specific Requirements | Risk Mitigation Value |
|---|---|---|---|
| Document Audit | Request ISO 13485:2016 + CE Technical File (including clinical evaluation report) | Confirm alignment with EU MDR Annex XIV Part A (Post-Market Surveillance) and IEC 60601-1-11 for home-use scenarios | High (Prevents shipment rejection at EU customs) |
| Factory Validation | Conduct 3rd-party audit via SGS/TÜV; verify production line calibration logs | Validate AI algorithm validation records (critical for IOS accuracy claims) | Critical (Avoids liability for non-conforming devices) |
| Market Surveillance | Cross-reference EUDAMED database; check FDA Establishment Registration | Confirm UDI compliance per China NMPA Rule 2025-07 | Medium-High (Prevents future recall liabilities) |
Step 2: Negotiating MOQ – Strategic Flexibility in Volatile Markets
Fixed high-volume MOQs create inventory obsolescence risks as IOS technology evolves rapidly (average 18-month product cycle). Implement dynamic ordering frameworks:
| Negotiation Strategy | 2026 Best Practice | Financial Impact |
|---|---|---|
| Phased Volume Commitment | Negotiate 60% base MOQ with 40% “flexible allocation” for new models (e.g., 20 units/base model + 10 units for next-gen) | Reduces obsolescence risk by 35-50%; preserves cash flow |
| Component-Level MOQ | Split orders: Scanner body (higher MOQ) vs. disposable tips/sensors (lower MOQ) | Optimizes inventory turnover; accommodates regional demand variances |
| Distributor Safeguards | Include “technology sunset clause” requiring 12-month advance notice before EOL | Prevents forced inventory write-downs; ensures migration path |
Step 3: Shipping & Logistics – DDP as the 2026 Standard
FOB terms increasingly expose buyers to hidden costs in China’s 2026 logistics environment (port congestion fees, carbon surcharges, customs valuation disputes).
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Base freight only; +18-25% hidden costs (THC, DOC, CIC) | Buyer liable for port delays, customs clearance errors | Avoid for IOS shipments; use only for pilot orders |
| DDP (Delivered Duty Paid) | All-inclusive pricing (freight, duties, VAT, last-mile) | Supplier bears all transit risks until clinic/distributor warehouse | Mandatory for volume orders; ensures landed cost certainty |
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Regulatory Excellence: ISO 13485:2016 certified factory with EU MDR-compliant CE Technical Files for all IOS models (NB ID: 0123). Full NMPA Class II registration for Chinese market distribution.
- MOQ Innovation: 5-unit base MOQ for flagship CJ-Scan Pro 2026 IOS with flexible allocation for AI software updates. Distributor-exclusive “Technology Refresh Program” included.
- DDP Guarantee: All shipments include carbon-neutral DDP terms with IoT environmental monitoring and guaranteed 22-day door-to-door delivery to EU/NA hubs.
- Vertical Integration: 19 years of in-house R&D (72 patents) for scanner optics and AI processing – eliminates component supply chain vulnerabilities.
Direct Factory Engagement:
Email: [email protected] | WhatsApp: +86 15951276160
Facility: 1288 Hengfeng Road, Baoshan District, Shanghai 200949, China
Factory audit appointments available with 72h notice
Conclusion
Successful IOS sourcing from China in 2026 requires shifting from transactional procurement to strategic partnership validation. Prioritize suppliers with demonstrable regulatory agility, flexible commercial terms aligned with technology cycles, and transparent DDP logistics. Shanghai Carejoy exemplifies this evolved model through its vertically integrated manufacturing, regulatory foresight, and distributor-centric commercial frameworks – making it a benchmark for risk-mitigated sourcing in the digital dentistry era.
Disclaimer: This guide reflects Q1 2026 regulatory and market conditions. Verify all requirements with local authorities prior to procurement. Global Dental Sourcing Advisory Group holds no financial interest in referenced entities.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Best Practices for Selecting a Dental Intraoral Scanner
Frequently Asked Questions: Purchasing the Best Dental Scanner in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a dental scanner for international or multi-location clinics? | Dental scanners typically operate on a wide input voltage range of 100–240V AC, 50/60 Hz, making them compatible with global power standards. However, confirm that the included power adapter or docking station supports your region’s voltage and plug type. For clinics in areas with unstable power, consider models with built-in surge protection or optional voltage stabilizers to prevent hardware damage. |
| 2. Are critical spare parts (e.g., scan tips, sensors, handpiece cables) readily available, and what is the average lead time? | Ensure the manufacturer or distributor maintains an accessible inventory of high-wear components such as scan tips, LED modules, and handpiece connectors. Leading OEMs in 2026 offer global logistics networks with average spare parts delivery within 3–7 business days. Opt for suppliers with regional warehouses or authorized service hubs to minimize downtime. Extended service agreements often include priority spare parts access. |
| 3. What does the standard installation process involve, and is on-site technician support included? | Installation typically includes hardware setup, software calibration, network integration, and initial user training. Most premium scanner vendors in 2026 provide complimentary on-site or remote installation by certified technicians. Confirm whether the package includes DICOM/EMR integration, CAD software licensing, and compatibility testing with existing lab workflows or milling systems. |
| 4. What is the standard warranty coverage for dental scanners, and are accidental damages included? | The standard warranty for high-end dental scanners in 2026 is typically 2 years, covering manufacturing defects and sensor malfunctions. Accidental damage (e.g., drops, liquid exposure) is generally excluded but can be added via an extended warranty or service plan. Verify coverage scope—some manufacturers now offer modular component warranties (e.g., 1 year on handpiece, 3 years on main processor). |
| 5. Can the warranty be extended, and what post-warranty service options are available for clinics planning long-term use? | Yes, most OEMs offer extendable warranties up to 5 years with optional coverage tiers. Post-warranty, clinics can enroll in annual service contracts that include preventive maintenance, software updates, priority repairs, and discounted spare parts. In 2026, several manufacturers also offer trade-in programs or technology refresh plans to support seamless upgrades. |
© 2026 Professional Dental Equipment Guide | For Authorized Distributors & Clinical Procurement Teams
Need a Quote for Best Dental Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


