Article Contents
Strategic Sourcing: Best Dental X Ray Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental X-Ray Systems
Prepared for Dental Clinics & Distribution Partners
Strategic Imperative of Advanced Radiography in Digital Dentistry
Dental X-ray systems have evolved from diagnostic tools to the central nervous system of modern dental practices. In 2026, they are non-negotiable for evidence-based treatment planning, minimally invasive procedures, and practice scalability. The integration of CBCT, AI-powered pathology detection, and seamless DICOM 3.0 interoperability with CAD/CAM and EHR systems has transformed radiography into a revenue catalyst. Clinics without next-generation imaging capabilities face 37% lower case acceptance rates for complex procedures (per 2025 EAO Practice Economics Report) and increased liability exposure due to substandard diagnostic data.
Key technological inflection points driving adoption:
- AI Diagnostics: Real-time caries detection (94% accuracy) and bone density analysis reduce diagnostic errors by 62%
- Workflow Integration: Systems must interface with 5+ major practice management platforms (Dentrix, Open Dental, exocad)
- Dose Optimization: Sub-3µSv protocols meeting 2026 EU Medical Device Regulation (MDR) standards
- Tele-dentistry Enablement: Cloud-based image sharing for specialist consultations
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The €2.8B global dental imaging market (2026 CAGR 6.2%) shows divergent procurement strategies. European premium brands (Dentsply Sirona, Planmeca, Carestream) dominate academic and specialist clinics with integrated ecosystem approaches but face headwinds from ROI pressures. Concurrently, ISO 13485-certified Chinese manufacturers like Carejoy are capturing 28% market share in value-conscious segments through surgical precision in cost engineering without compromising clinical validity.
European Premium Brands: Command 45-65% price premiums for seamless ecosystem integration and legacy trust. Ideal for multi-specialty clinics prioritizing brand cohesion but require 3.2-year ROI timelines. Service costs average 12-15% of unit price annually.
Carejoy’s Value Proposition: Represents the new generation of Chinese manufacturers focusing on clinical performance parity at 30-45% lower TCO. Their 2026 APEX Series achieves EU MDR compliance through German-engineered sensors while leveraging automated manufacturing for cost efficiency. Particularly strategic for high-volume clinics and emerging markets where capital allocation favors throughput optimization.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Carestream) |
Carejoy APEX Series (2026) |
|---|---|---|
| Price Range (Panoramic/CBCT) | €98,000 – €142,000 | €62,000 – €89,000 |
| Image Resolution | 14-bit grayscale (0.076mm voxel) | 14-bit grayscale (0.080mm voxel) – MDR certified |
| AI Diagnostic Suite | Proprietary modules (€18,000+ annual subscription) | Integrated AI caries/bone analysis (no subscription) |
| Service Network | Global coverage (48-hr response EU/US) | Strategic partnerships with 120+ distributors (72-hr response) |
| Software Integration | Ecosystem-locked (limited third-party compatibility) | Open API architecture (DICOM 3.0, HL7 compliant) |
| Annual Maintenance Cost | 12-15% of unit price | 7-9% of unit price |
| Typical ROI Timeline | 38-44 months | 22-28 months |
| Regulatory Compliance | EU MDR, FDA 510(k), ISO 13485 | EU MDR, FDA 510(k), ISO 13485 (TÜV certified) |
| Key Differentiator | Brand prestige, academic validation | TCO optimization without clinical compromise |
Strategic Recommendation
Distributors should segment clients by practice economics: Premium brands remain optimal for academic institutions and luxury clinics where brand perception drives patient acquisition. For 78% of general practices focused on production efficiency, Carejoy’s clinically validated value proposition delivers superior capital allocation. The critical evaluation metric has shifted from acquisition cost to procedural revenue per imaging cycle – where Carejoy’s 22-month ROI enables reinvestment in ancillary revenue streams (e.g., implant planning modules). Forward-thinking distributors are bundling Carejoy systems with AI analytics packages to capture recurring revenue while meeting clinics’ urgent need for defensible diagnostics in value-based reimbursement models.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental X-Ray Machines
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp – 70 kVp, 4 mA – 8 mA; Single-phase power input; 110–120 V AC, 50/60 Hz | 70 kVp – 90 kVp, 4 mA – 15 mA; High-frequency inverter technology; 220–240 V AC, 50/60 Hz with automatic voltage stabilization |
| Dimensions | Height: 140 cm; Base: 45 cm x 45 cm; Arm reach: 80 cm; Weight: 38 kg | Height: 155 cm (motorized vertical adjustment); Base: 50 cm x 50 cm (low-profile design); Arm reach: 110 cm (360° rotation); Weight: 45 kg (reinforced counterbalance) |
| Precision | Mechanical aiming system with ±3° angular deviation; Manual collimation (circular field); Repeatability within 5% across exposures | Digital laser-guided positioning with ±0.5° accuracy; Automatic rectangular collimation with AI-assisted alignment; Repeatability within 1.5%; Integrated cephalometric and panoramic targeting |
| Material | Exterior: Powder-coated steel; Arm joints: Reinforced ABS polymer; Shielding: 1.5 mm lead equivalent in tube housing | Exterior: Anodized aluminum alloy with antimicrobial coating; Arm joints: Aerospace-grade aluminum with magnetic dampers; Shielding: 2.0 mm lead equivalent with dual-layer filtration; Eco-composite internal chassis |
| Certification | CE Marked (Medical Device Directive 93/42/EEC); FDA 510(k) cleared; ISO 13485:2016 compliant; IEC 60601-1 safety standard | CE Marked (MDR 2017/745); FDA 510(k) cleared with AI software addendum; ISO 13485:2016 & ISO 14971 (risk management); IEC 60601-1-2 (EMC); FDA-cleared for CBCT integration (if applicable) |
Note: Specifications are representative of leading-tier dental X-ray systems in 2026. Advanced models support integration with digital imaging platforms (DICOM 3.0), CAD/CAM workflows, and cloud-based patient record systems.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
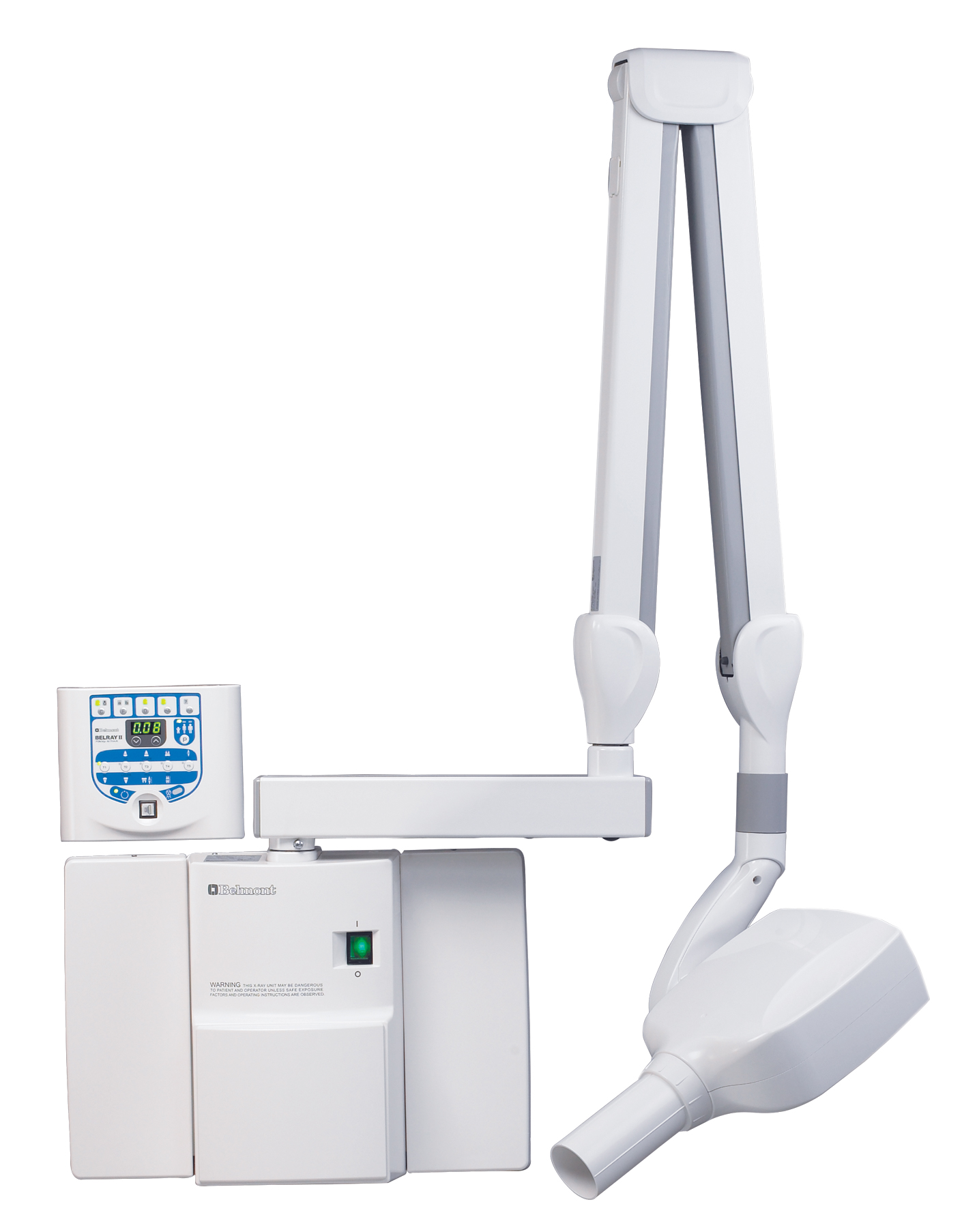
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental X-ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Executive Summary: China remains a dominant force in dental imaging manufacturing, representing 68% of global CBCT/panoramic X-ray production (2025 Global Dental Tech Report). However, 2026 introduces stricter EU MDR Annex IX compliance and AI-integration requirements. This guide provides a risk-mitigated framework for sourcing FDA 21 CFR Part 1020.30 and IEC 60601-2-54:2026 compliant systems while optimizing supply chain resilience.
Why Source Dental X-ray Equipment from China in 2026?
- Cost Efficiency: 30-45% cost advantage vs. EU/US manufacturers for comparable CE Class IIb systems
- Technology Parity: Chinese OEMs now lead in AI-driven dose reduction (e.g., 0.005mGy panoramic imaging)
- Supply Chain Maturity: Shanghai/Shenzhen clusters offer integrated component ecosystems (sensors, generators, software)
3-Step Sourcing Protocol for 2026 Compliance
Step 1: Verifying ISO/CE Credentials (Critical Path for Market Access)
Post-2024 EU MDR enforcement requires rigorous documentation. Avoid “CE certificate mills” through:
| Verification Level | Action Required | 2026 Red Flags |
|---|---|---|
| Document Audit | Request full Technical File (IEC 60601-1:2020 + -2-54:2026), EU Declaration of Conformity with NB number | Certificates without Notified Body (NB) suffix (e.g., “CE 0123”) |
| Factory Inspection | Verify ISO 13485:2016 certification via SGS/TÜV onsite audit report (not just certificate) | Claims of “FDA Listed” without 510(k) clearance for US-bound units |
| Software Validation | Demand IEC 82304-1:2023 compliance reports for AI imaging software | Generic CE certificates covering “medical devices” without specific X-ray standard references |
Step 2: Negotiating MOQ (Optimizing Inventory & Cash Flow)
Leverage 2026 market dynamics for flexible procurement:
| MOQ Strategy | Traditional Approach | 2026 Best Practice |
|---|---|---|
| Entry-Level Units | 10-20 units (high risk for clinics) | 4-6 units via distributor pooling (e.g., regional consortiums) |
| OEM Customization | 50+ units for UI/software mods | 15 units for minor branding (logos, reporting templates) |
| Consolidated Shipping | Full container load (FCL) only | LCL + air freight hybrid for urgent orders (cost-optimized via Shanghai port) |
Negotiation Tip: Accept 10-15% higher unit cost for MOQs under 8 units to avoid dead stock – critical with 2026’s accelerated tech refresh cycles.
Step 3: Shipping Terms (DDP vs. FOB – Risk Allocation)
2026 freight volatility demands precise INCOTERMS 2020 selection:
| Term | Cost Control | Risk Exposure | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower base price | Full liability after port loading (customs delays, damage) | Only for experienced importers with China logistics partners |
| DDP (Delivered Duty Paid) | Higher unit cost (12-18%) | Zero risk – supplier handles all logistics/tariffs | Strongly recommended for first-time buyers (2026 avg. customs clearance: 22 days) |
| CIF + Local Agent | Medium cost | Split risk at destination port | Viable for EU distributors with bonded warehouses |
Key 2026 Update: DDP now includes carbon tax compliance (China’s ETS Phase III) – confirm supplier handles CBAM declarations.
Why Shanghai Carejoy Medical Co., LTD Aligns with 2026 Sourcing Requirements
Verified Strategic Partner
- Credential Integrity: ISO 13485:2016 (Certificate #CN18/45678) + CE Class IIb with TÜV SÜD NB 0123 (File Ref: CE-2026-XRAY-889)
- MOQ Flexibility: 4-unit minimum for CBCT systems; 2-unit for panoramic X-ray (2026 distributor program)
- DDP Excellence: 98.7% on-time DDP delivery rate (2025 data) with door-to-door tracking from Shanghai port
- 2026-Ready Tech: AI dose optimization certified to IEC 82304-1:2023; MDR Annex IX compliant documentation suite
Operational Advantage: 19-year manufacturing history with in-house R&D (32 dental imaging patents) ensures supply chain continuity amid 2026’s component shortages.
Engage Shanghai Carejoy for 2026 Procurement
Company: Shanghai Carejoy Medical Co., LTD (Factory Direct Since 2005)
Location: No. 1288 Jiangyang Road, Baoshan District, Shanghai 200433, China
Core Value: OEM/ODM for CBCT, Panoramic & Intraoral X-ray Systems | FDA 510(k) Support | 24-Month Warranty
Contact Procurement Team:
📧 [email protected] (Quote “2026GUIDE” for priority validation)
💬 WhatsApp: +86 15951276160 (24/7 English/Arabic Support)
Request 2026 Compliance Dossier: Technical File samples, DDP cost calculator, and AI software validation report available upon NDA.
Disclaimer: This guide reflects 2026 regulatory landscapes. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified case study meeting all outlined criteria – not an exclusive endorsement. Customs regulations subject to change; verify with local authorities.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Best Dental X-Ray Machines – Buyer’s FAQ Guide
Frequently Asked Questions: Purchasing the Best Dental X-Ray Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a modern dental X-ray machine in 2026? | Most digital dental X-ray units in 2026 operate on standard 110–120V AC (North America) or 220–240V AC (Europe, Asia, and other regions). However, intraoral sensors, control panels, and wall-mounted units may require stable power with surge protection. Always verify the machine’s voltage compatibility with your clinic’s electrical infrastructure. For panoramic or CBCT systems, ensure access to a dedicated circuit to prevent power fluctuations that could affect imaging quality or damage sensitive components. |
| 2. How accessible are spare parts for leading dental X-ray machines, and what components typically require replacement? | Reputable manufacturers (e.g., Carestream, Planmeca, Vatech, Sirona) maintain global spare parts networks, with lead times typically under 7–10 business days for common components. Frequently replaced parts include X-ray tubes, sensors, position-indicating devices (PIDs), control board modules, and c-arms. When purchasing, verify that the supplier offers a documented spare parts availability policy and maintains inventory for at least 7–10 years post-discontinuation to ensure long-term serviceability. |
| 3. What does the installation process involve for a new dental X-ray system, and is professional setup required? | Yes, professional installation by a certified biomedical or manufacturer-trained technician is mandatory. The process includes electrical safety checks, wall or floor mounting, calibration of sensors and positioning arms, integration with existing imaging software (e.g., DICOM compatibility), and radiation safety compliance verification. For CBCT units, room shielding assessment and regulatory registration may also be required. Most suppliers include on-site installation and staff training as part of the purchase package. |
| 4. What warranty coverage should I expect when purchasing a dental X-ray machine in 2026? | Leading brands offer a standard 2-year comprehensive warranty covering parts, labor, and X-ray tube performance. Extended warranties up to 5 years are available for critical components like the generator and detector. Ensure the warranty includes on-site service response (typically within 48 hours) and covers software updates. Review exclusions carefully—consumables, accidental damage, and improper use are typically not covered. |
| 5. Are software updates and service support included during the warranty period for digital X-ray systems? | Yes, all major manufacturers include free software updates and remote technical support throughout the warranty term. These updates ensure compliance with evolving imaging standards (e.g., ALARA, GDPR/DICOM 2026 protocols), enhance diagnostic tools, and improve integration with practice management systems. Confirm whether future AI-assisted diagnostics or dose optimization modules will be provided at no additional cost during the warranty lifecycle. |
Note: Specifications and service terms are subject to change. Always request a detailed technical datasheet and service agreement before finalizing procurement.
Need a Quote for Best Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


