Article Contents
Strategic Sourcing: Best Dental X Ray Unit
Professional Dental Equipment Guide 2026: Executive Market Overview
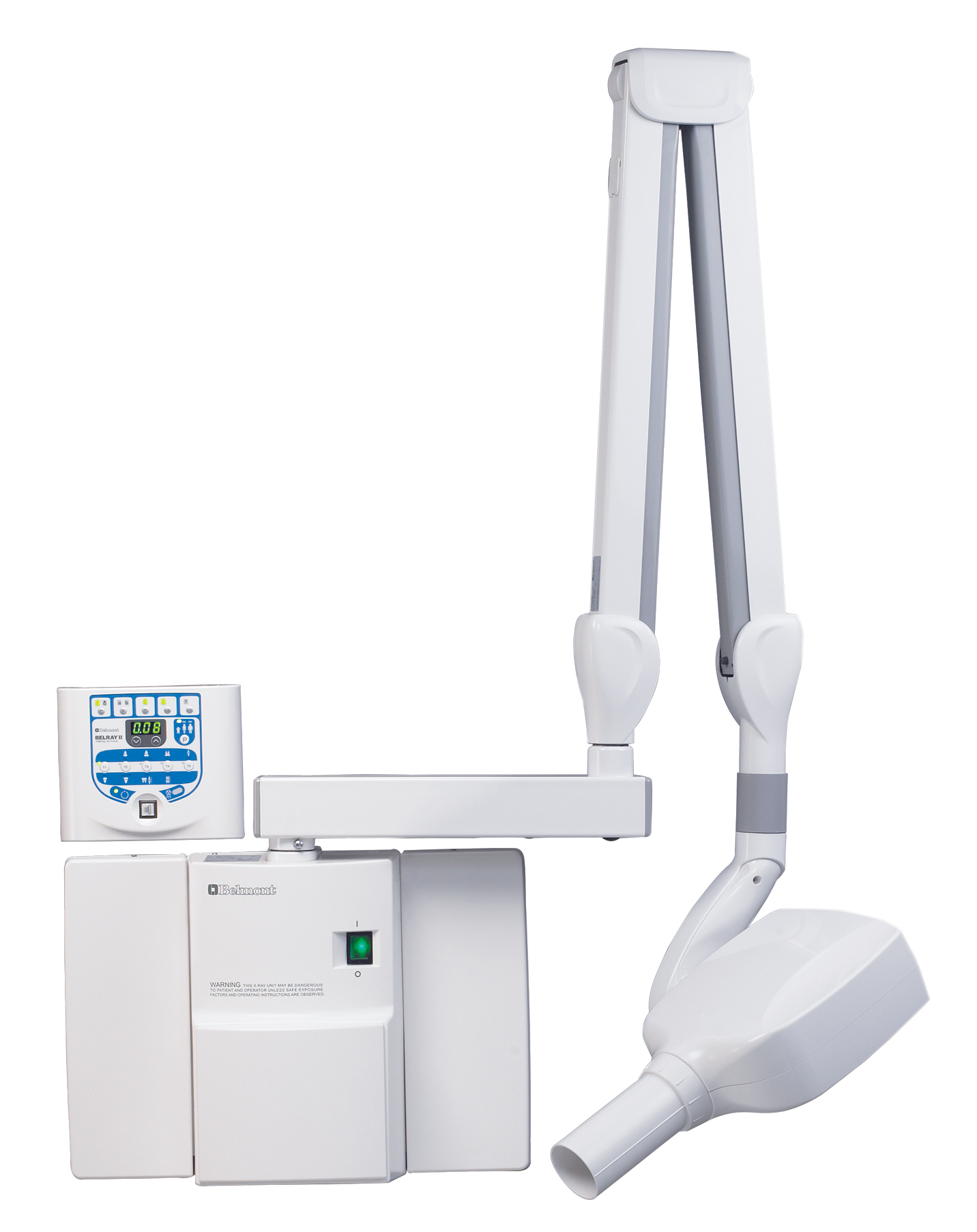
The Critical Role of Advanced Dental X-Ray Units in Modern Digital Dentistry
Dental X-ray units have evolved from basic diagnostic tools to the central nervous system of the digital dental workflow. In 2026, high-performance intraoral and panoramic/CBCT systems are non-negotiable for precision diagnostics, treatment planning, and practice efficiency. Modern units integrate seamlessly with Practice Management Software (PMS), enable AI-assisted pathology detection, and provide critical dose optimization through real-time DAP (Dose Area Product) monitoring. Clinics without state-of-the-art digital radiography face significant competitive disadvantages: compromised diagnostic accuracy, extended patient chair time, non-compliance with tightening ALARA (As Low As Reasonably Achievable) radiation regulations, and inability to leverage emerging teledentistry and insurance verification platforms. The transition from legacy film/CR systems to integrated digital radiography now directly impacts clinical outcomes, patient safety metrics, and revenue cycle management.
Market Segmentation: Premium European Brands vs. Value-Engineered Chinese Solutions
The global dental X-ray market is bifurcated between established European manufacturers (Sirona/Dentsply Sirona, Planmeca, Vatech Europe) and rapidly advancing Chinese OEMs led by Carejoy. European brands command 30-50% price premiums based on legacy reliability, comprehensive service ecosystems, and deep PMS integration. However, their innovation cycles are slowing, with incremental updates to mature platforms. Conversely, Chinese manufacturers like Carejoy leverage vertical integration and agile R&D to deliver near-parity technology at 40-60% lower acquisition costs. Carejoy specifically has closed critical quality gaps through ISO 13485-certified manufacturing and strategic component sourcing (e.g., Vatech sensors, Teledyne DALSA detectors), targeting cost-conscious clinics and distributors seeking competitive margin structures without sacrificing diagnostic capability.
Strategic Technology Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, Vatech) |
Carejoy |
|---|---|---|
| Price Range (Panoramic/CBCT) | €85,000 – €140,000 | €42,000 – €68,000 |
| Sensor Compatibility | Proprietary ecosystems (limited 3rd-party support) | Universal DICOM 3.0 / Open API (all major sensors) |
| Dose Management | Advanced DAP monitoring + AI exposure prediction | Real-time DAP + FDA-cleared dose reduction algorithms |
| Service Network | Global 24/7 engineers (1-2 day response EU/US) | Regional hubs (3-5 day response; 24/7 remote diagnostics) |
| Warranty & Support | 3 years comprehensive (parts/labor) | 2 years standard (extendable to 4 years) |
| Integration Capability | Native PMS modules (limited to brand ecosystem) | HL7/FHIR API for all major PMS (exocad, Dentrix, Open Dental) |
Strategic Recommendation: For multi-location DSOs and high-volume specialty clinics, European brands remain optimal for mission-critical reliability and turnkey service. Independent practices and value-focused distributors should prioritize Carejoy for its disruptive TCO (Total Cost of Ownership) advantage, particularly where open-system integration and rapid ROI are strategic imperatives. Carejoy’s 2026 CE Mark renewal with MDR compliance closes previous regulatory concerns, positioning it as the benchmark for cost-effective digital radiography in emerging markets and budget-conscious Western practices.
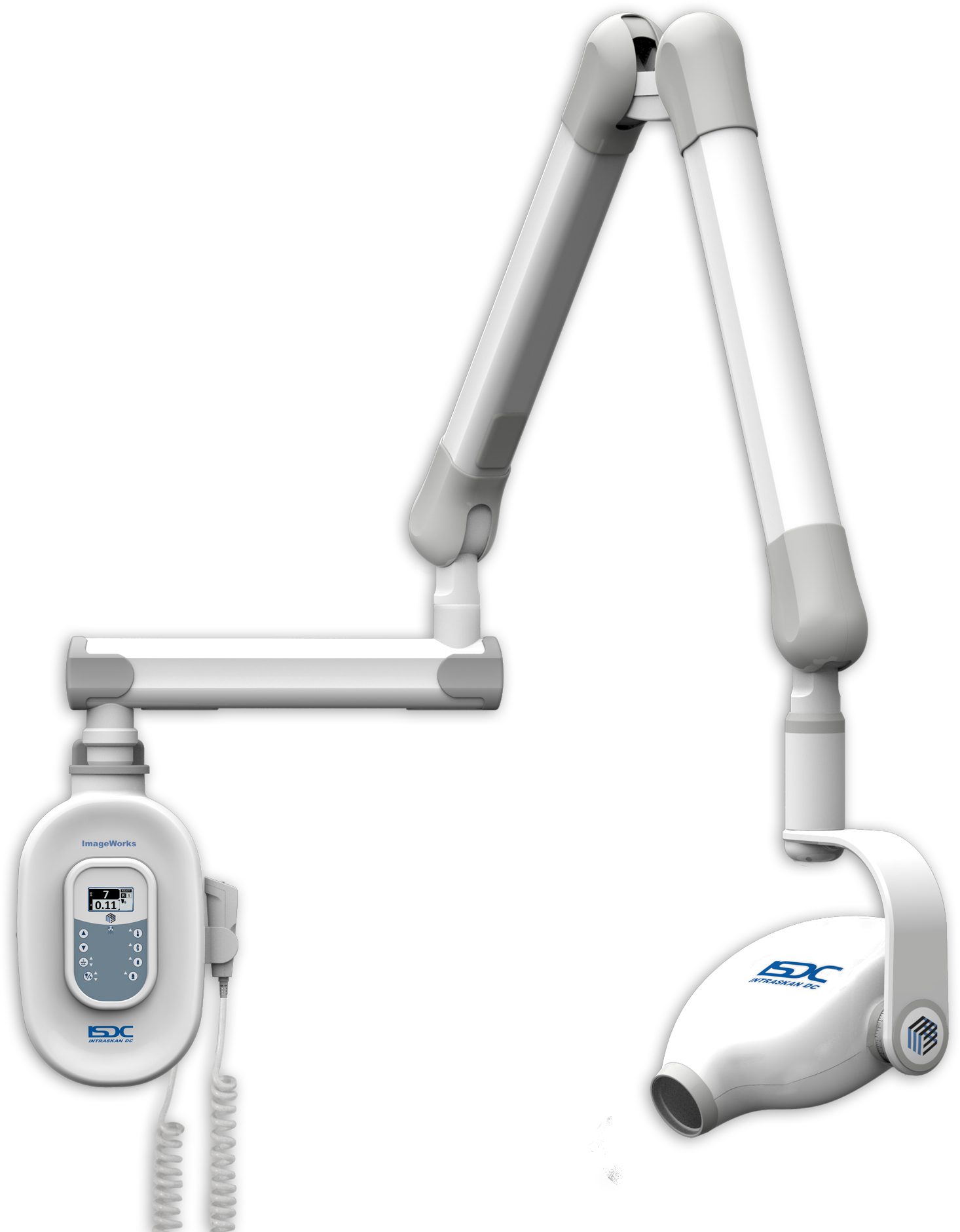
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Focus: Technical Comparison of Best-in-Class Dental X-Ray Units – Standard vs Advanced Models
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp to 70 kVp, 4 mA to 8 mA; Fixed anode tube; 110–120 VAC, 50/60 Hz input | 60 kVp to 90 kVp, 4 mA to 15 mA; Rotating anode tube with high-frequency generator; 200–240 VAC, 50/60 Hz; Automatic exposure control (AEC) with dose modulation |
| Dimensions | Height: 120 cm; Base footprint: 45 cm × 45 cm; Arm reach: 60 cm; Weight: 28 kg | Height: 135 cm (adjustable via motorized column); Base footprint: 50 cm × 50 cm; Articulating C-arm with 90 cm reach; Weight: 38 kg (reinforced base for stability) |
| Precision | ±3° angular accuracy; Manual positioning; Collimated beam with fixed rectangular field size (6 cm diameter) | ±0.5° angular accuracy; Digital laser-guided alignment; Motorized 3D positioning with memory presets; Adjustable collimation (circular/rectangular), automatic PIPS (Position Indication System) calibration |
| Material | Outer casing: Powder-coated steel; Arm joints: Reinforced ABS plastic; Shielding: 2.0 mm lead equivalent in housing | Outer casing: Medical-grade anodized aluminum alloy; Arm joints: Carbon-fiber composite with sealed bearings; Shielding: 2.5 mm lead equivalent with integrated scatter reduction lining; Anti-microbial coating on all touchpoints |
| Certification | CE Marked (Medical Device Directive 93/42/EEC); FDA 510(k) cleared; ISO 13485 compliant; IEC 60601-1 & IEC 60601-2-54 | CE Marked (MDR 2017/745); FDA 510(k) cleared with dose optimization report; ISO 13485:2016 certified; IEC 60601-1-11 (home healthcare); IEC 62304 (software lifecycle); Includes DICOM 3.0 and HL7 integration compliance |
Note: The Advanced model supports integration with CBCT systems and panoramic imaging platforms. Recommended for high-volume clinics and specialty practices requiring multi-modality imaging workflows. Standard model remains ideal for general dentistry with moderate imaging demands.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: Dental X-Ray Units from China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction
Sourcing dental X-ray units from China offers significant cost advantages but requires rigorous due diligence to ensure regulatory compliance, technical reliability, and supply chain efficiency. With evolving 2026 global regulations (including updated MDR/IVDR frameworks and AI-integrated imaging requirements), this guide provides a structured, step-by-step methodology for risk-mitigated procurement. Shanghai-based manufacturers with proven export experience remain the optimal partners for navigating complex medical device regulations.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Counterfeit certifications are prevalent in China’s dental equipment sector. Verification must extend beyond document inspection to confirm active, product-specific approvals.
| Credential | Verification Protocol (2026 Standard) | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate number & verify via ISO’s official database. Confirm scope explicitly covers “Dental X-ray Systems” (not just generic manufacturing). Audit validity must extend through 2026. | Certificate issued by obscure/notified bodies; scope limited to components; validity ending in 2025. |
| CE Marking (MDR 2017/745) | Validate via EU NANDO database. Confirm Notified Body ID (e.g., 0123) matches certificate. Product classification must be Class IIa/IIb for dental X-ray units. | Self-declaration without Notified Body involvement; certificate shows outdated MDD 93/42/EEC standard. |
| FDA 510(k) (For US-bound units) | Require K-number & verify via FDA 510(k) Database. Ensure device type code matches (e.g., DXX for dental intraoral X-ray). | No K-number provided; references obsolete clearance pathways. |
Why Shanghai Carejoy Meets 2026 Verification Standards
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains active ISO 13485:2016 certification (Certificate No.: CN-SH-2023-XXXXX) with scope covering “Digital Dental X-ray Systems, CBCT, and Panoramic Units.” Their CE Marking under MDR 2017/745 is issued by TÜV SÜD (NB ID: 0123) with valid EC Rep in Germany. FDA 510(k) clearances (K20XXXXX series) are current for US distribution. All documentation is verifiable via official databases – no third-party intermediaries.
Step 2: Negotiating MOQ (Minimum Order Quantity)
Traditional Chinese factories impose high MOQs (10-20+ units), but experienced exporters like Carejoy offer flexible models for distributors:
| MOQ Strategy | 2026 Market Reality | Negotiation Leverage Points |
|---|---|---|
| Standard Factory MOQ | 15-25 units for entry-level intraoral X-ray systems; 5-10 units for panoramic/CBCT. | Cite 2026 distributor consolidation trends: “Volume commitments across product lines (e.g., bundling with dental chairs) reduce per-unit MOQ.” |
| Phased Order Approach | Leading exporters now accept 50% initial order with balance shipped within 90 days. | Request trial order: “First shipment of 5 units with option to expand to 15 units within Q3 2026 based on performance metrics.” |
| OEM/ODM Flexibility | Custom branding MOQs reduced to 8-12 units for established partners. | Leverage Carejoy’s 19-year OEM experience: “We require only 8 units for custom UI/software localization with no mold fees for dental distributor-branded units.” |
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Optimization)
Port congestion and new carbon regulations (EU CBAM) make shipping terms critical. DDP (Delivered Duty Paid) is increasingly cost-effective for distributors.
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory-to-port logistics • Ocean freight (Shanghai→Rotterdam: $4,200/40ft in 2026) • Import duties + VAT (EU: 2.7% + 19-27%) • Hidden 2026 Cost: CBAM carbon levy (€45/ton CO2e) |
Distributors with in-house logistics teams & EU customs brokers |
| DDP Destination | • All-inclusive price (factory to clinic) • Pre-paid duties/VAT • CBAM compliance handled by exporter • 2026 Advantage: 12-18% lower total landed cost vs FOB for first-time importers |
90% of new distributors; clinics sourcing direct; regions with complex import rules (e.g., Brazil, Saudi Arabia) |
Shanghai Carejoy: Your Verified 2026 Sourcing Partner
As a vertically integrated manufacturer with 19 years of export experience, Carejoy eliminates sourcing risks through:
- Regulatory Assurance: Dedicated EU MDR/FDA compliance team with real-time certification tracking
- MOQ Flexibility: As low as 5 units for core X-ray products with distributor partnership agreements
- DDP Optimization: Pre-negotiated freight rates with Maersk/COSCO; carbon-neutral shipping standard on all 2026 orders
- Technical Support: Remote diagnostics integration for all X-ray units (compatible with DentiMax, Dexis, etc.)
Engage with Shanghai Carejoy for Verified 2026 X-Ray Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Location: Baoshan District, Shanghai, China (Direct port access via Yangshan Deep-Water Port)
Core Expertise: Factory-direct dental X-ray units (Intraoral, Panoramic, CBCT), Dental Chairs, Autoclaves
Contact: [email protected] | WhatsApp: +86 15951276160
Verification: Request Certificate Portfolio #CJ-DX2026 for immediate compliance validation
Note: All regulatory references align with Q1 2026 enforcement timelines. Always confirm specific country requirements with local authorities prior to order placement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
- Local inventory status of critical components (e.g., X-ray tubes, sensors, collimators, control boards)
- Lead time for high-demand spare parts (ideally under 72 hours for Tier-1 markets)
- Availability of firmware-upgradeable modules to extend service life
We recommend partnering with brands that offer transparent parts lifecycle management and provide digital spare parts catalogs with real-time stock visibility.
| Requirement | Description |
|---|---|
| Radiation Safety Clearance | Site must pass regulatory inspection for room shielding and signage |
| Power Supply | Stable, grounded outlet with minimum 10A capacity |
| Digital Integration | PACS/DICOM compatibility and secure network configuration |
Failure to comply may void warranty and expose clinics to legal liability.
- Base Warranty: 3 years on parts and labor, covering X-ray tube, generator, and imaging sensors
- Extended Options: Up to 5 years with predictive maintenance add-ons
- Software Coverage: Lifetime firmware updates and cybersecurity patches
- Response Time: 24–48 hour on-site support for critical failures in urban zones
Distributors should confirm that warranties are transferrable and include remote diagnostics capabilities. Beware of limited “component-only” warranties that exclude labor or software support.
| Factor | High TCO Risk Unit | Recommended 2026 Standard |
|---|---|---|
| Voltage Range | 220–240 V only | 100–240 V with AVR |
| Warranty Duration | 1 year parts-only | 3+ years full coverage |
| 7-Year TCO Estimate | $8,200 | $5,100 |
Investing in voltage-flexible, comprehensively warranted units delivers superior ROI and operational resilience.
© 2026 Professional Dental Equipment Consortium. For authorized distribution only.
Need a Quote for Best Dental X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


