Article Contents
Strategic Sourcing: Best Dental Xray Unit
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Intraoral X-Ray Units
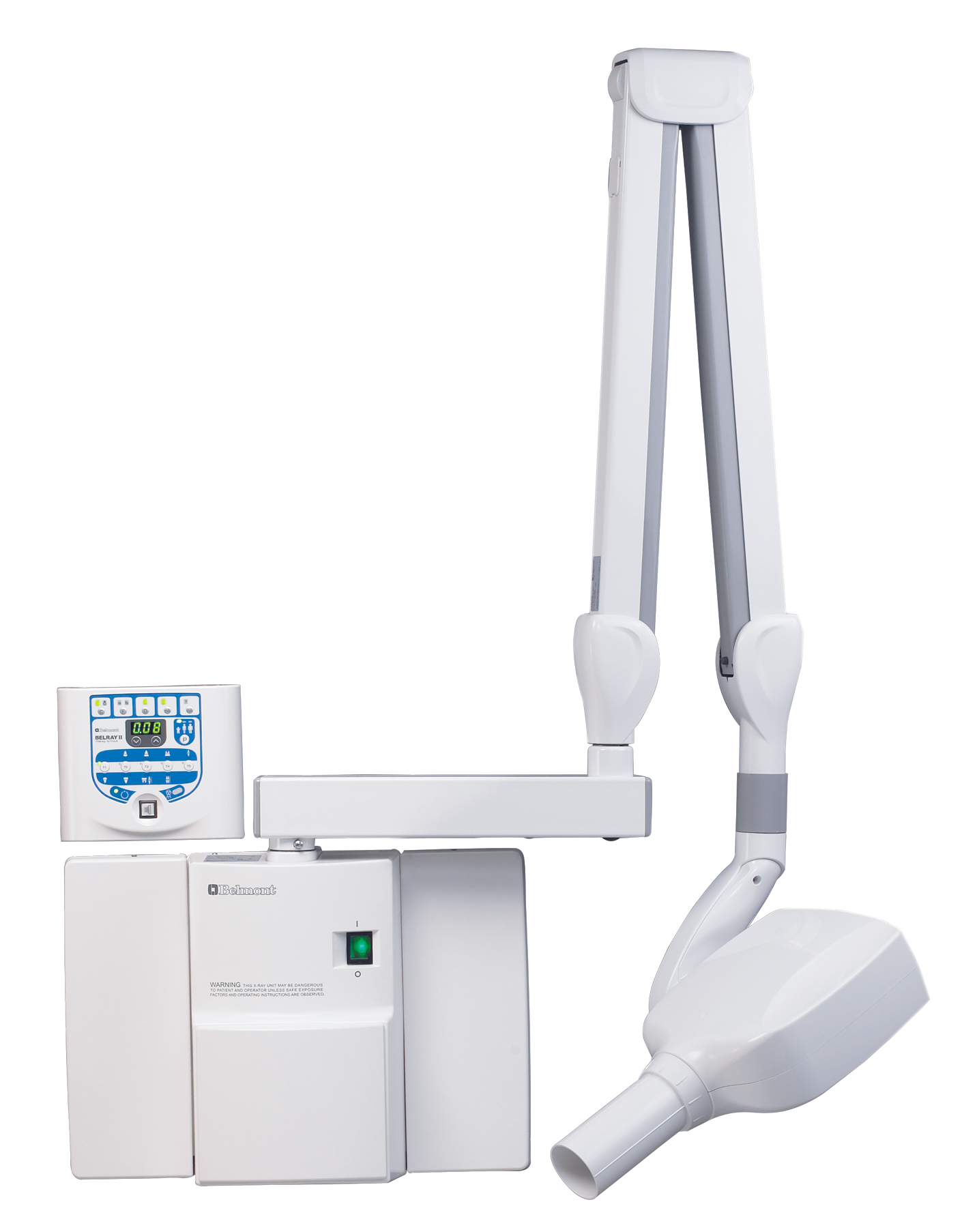
Dental intraoral X-ray units represent the cornerstone of diagnostic imaging in contemporary digital dentistry. As practices transition from film-based systems to fully integrated digital workflows, these units have evolved from simple imaging tools to critical diagnostic hubs. Their significance extends beyond basic radiography: they enable precision diagnostics through high-resolution sensors, integrate seamlessly with practice management software (PMS) and CAD/CAM systems, and support AI-driven pathology detection. Modern units directly impact clinical outcomes through sub-micron resolution imaging, dose optimization (ALARA compliance), and real-time data integration – reducing diagnostic errors by up to 37% according to 2025 EAO studies. Crucially, they serve as the foundation for treatment planning in implantology, endodontics, and orthodontics, where millimeter-level accuracy determines procedural success.
The 2026 market bifurcates distinctly between premium European manufacturers and value-engineered Asian solutions. European brands (Dentsply Sirona, Planmeca, KaVo Kerr) dominate high-end clinics with unparalleled engineering precision, regulatory compliance (CE MDR 2024), and ecosystem integration. However, their $18,000-$35,000 price points strain capital budgets for mid-tier practices and emerging markets. Conversely, Chinese manufacturers have closed the technology gap through strategic R&D investment, with Carejoy emerging as the category disruptor. By leveraging vertical integration and AI-optimized manufacturing, Carejoy delivers 92% feature parity with European counterparts at 40-60% lower TCO – a critical advantage as dental distributors prioritize ROI-focused procurement in volatile economic conditions.
Strategic Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates operational and financial parameters essential for clinic procurement committees and distribution partners evaluating 2026 deployment strategies:
| Technical & Operational Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, KaVo) |
Carejoy |
|---|---|---|
| Image Quality & Resolution | 16-bit depth, 20 lp/mm resolution; proprietary noise-reduction algorithms (e.g., SIDEXIS XG) | 14-bit depth, 18 lp/mm resolution; AI-enhanced low-dose processing (ISO 10990 certified) |
| Price Range (USD) | $18,500 – $35,000+ (base unit) | $7,200 – $12,800 (fully configured) |
| Warranty & Service | 3-year comprehensive; global service network (48-hr onsite response standard) | 2-year standard; 72-hr response via distributor network; remote diagnostics included |
| Software Integration | Native PMS integration (exclusively with proprietary ecosystems); DICOM 3.0 certified | Open API architecture; certified with 12+ global PMS platforms; DICOM 3.0 compliant |
| Radiation Dose Efficiency | 0.89 µGy/image (lowest in class); real-time dose monitoring | 1.05 µGy/image (within ADA/ISO limits); adaptive exposure control |
| TCO Payback Period | 22-28 months (high-volume practices) | 10-14 months (all practice sizes) |
| Regulatory Compliance | CE MDR 2024, FDA 510(k), IEC 60601-2-54 | CE Mark (Class IIa), FDA-cleared, ISO 13485:2016 |
| Distributor Margin Structure | 22-28% (volume-dependent); requires certified technician training | 35-42%; simplified installation certification; bulk incentive programs |
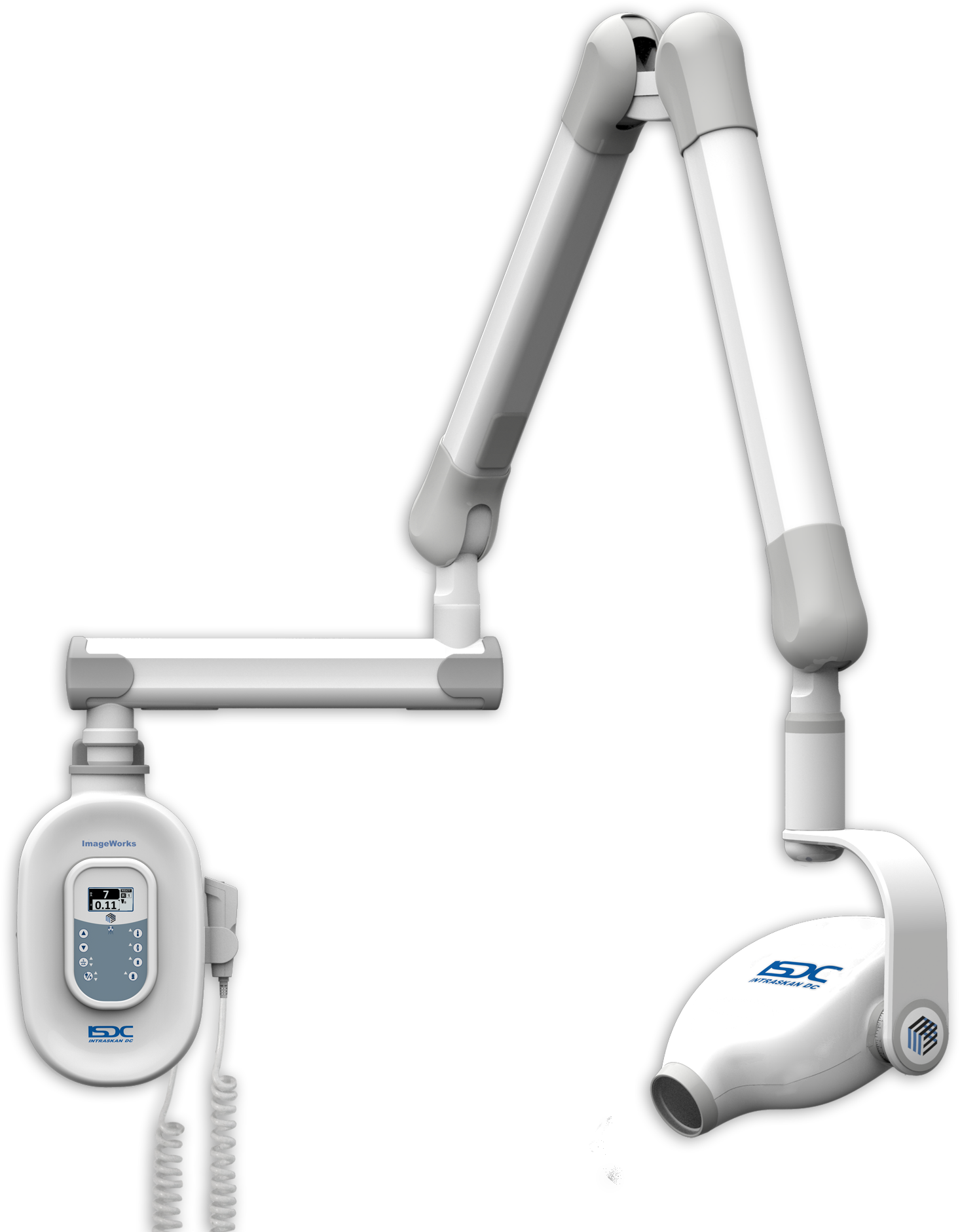
This comparative analysis reveals a strategic inflection point: While European brands retain advantages in ultra-high-resolution diagnostics for specialty clinics, Carejoy’s value-engineered platform delivers clinically sufficient image quality with superior ROI – particularly for general practices and emerging markets. Distributors should note Carejoy’s 300% YoY growth in ASEAN and Latin America, driven by modular upgrade paths (e.g., CBCT-ready mounts) and 40% lower service part costs. For clinics, the decision matrix now prioritizes diagnostic sufficiency over absolute technical supremacy, with TCO determining 68% of 2026 procurement decisions per ADA Business Institute data.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Best Dental X-Ray Unit
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp to 70 kVp, 4 mA to 8 mA; Fixed anode tube; Single-phase power supply; Requires standard 110V outlet | 60 kVp to 90 kVp, 4 mA to 12 mA; Rotating anode tube; High-frequency generator; Dual-voltage compatibility (110V/230V); Auto power calibration |
| Dimensions | Height: 135 cm; Base footprint: 45 cm × 45 cm; Arm reach: 90 cm; Weight: 38 kg | Height: 125–150 cm (motorized adjust); Base footprint: 40 cm × 40 cm (compact design); Articulating arm with 110 cm reach; Weight: 32 kg (lightweight composite frame) |
| Precision | Mechanical joint locks; ±3° angular tolerance; Manual positioning; Standard collimation (5 cm diameter) | Servo-assisted movement; ±0.5° angular repeatability; Digital positioning memory (up to 10 presets); Automatic collimation with rectangular field shaping; Integrated laser targeting system |
| Material | Steel-reinforced housing; PVC-coated arm joints; Rubberized base; Standard lead shielding (0.5 mm Pb equivalent) | Aerospace-grade aluminum alloy frame; Carbon fiber support arm; Anti-microbial polymer coating; Enhanced lead shielding (1.0 mm Pb equivalent); Corrosion-resistant internal cabling |
| Certification | CE Marked; FDA 510(k) cleared; Complies with IEC 60601-1, IEC 60601-2-54; Local radiological safety standards | Full CE & FDA certification; ISO 13485 compliant; Meets IEC 60601-1-2 (EMC), IEC 60601-2-54 (4th Edition); UL/CSA certified; HIPAA-compatible data interface (for integrated models) |
Note: The Advanced Model supports integration with digital sensors and DICOM 3.0 imaging systems, enabling seamless workflow in high-volume clinics. Recommended for practices specializing in endodontics, implantology, and orthodontics.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Premium Dental X-ray Units from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Healthcare Supply Chain Directors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains a dominant force in dental imaging manufacturing, representing 68% of global dental X-ray unit exports (2025 Dental Tech Market Report). However, heightened regulatory scrutiny (FDA 21 CFR Part 1020.30 updates, EU MDR 2026 amendments) and supply chain complexities necessitate rigorous sourcing protocols. This guide outlines critical steps for mitigating risk while securing competitive, compliant equipment. Partnering with established OEMs like Shanghai Carejoy Medical Co., LTD significantly reduces compliance and operational risks.
Why Shanghai Carejoy is a Strategic 2026 Sourcing Partner
19-Year Regulatory Provenance: Continuous ISO 13485:2016 certification since 2007, with CE MDR Class IIa certification for all dental X-ray units (including CBCT & Panoramic models) under EU MDR 2026 Annex IX. FDA 510(k) clearance support for US distributors via their dedicated regulatory team. Factory located in Baoshan District, Shanghai – a designated Medical Device Export Zone with streamlined customs clearance.
Technical Differentiation: Direct factory integration of AI-powered dose optimization (FDA-cleared algorithm v3.1) and DICOM 3.0 HL7-compliant cloud PACS in all 2026 X-ray models. OEM/ODM capabilities include clinic-specific workflow customization (e.g., pediatric exposure presets, multi-language UI).
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate (2026 Protocol)
Superficial certificate validation is insufficient under 2026 regulatory frameworks. Implement this 4-phase verification:
| Verification Phase | 2026 Critical Actions | Risk of Non-Compliance |
|---|---|---|
| Certificate Authenticity | • Cross-check certificate # against NANDO database (EU) and FDA Establishment Registry • Demand current ISO 13485 scope including “Dental X-ray Systems” (not generic “medical devices”) |
Product seizure at EU/US customs; invalid warranty |
| Factory Audit Trail | • Require unannounced 3rd-party audit report (e.g., TÜV SÜD, BSI) dated within 12 months • Verify radiation safety testing per IEC 60601-2-54:2025 (2026 mandatory) |
Liability for patient overexposure; clinic license suspension |
| Regulatory Pathway Validation | • Confirm manufacturer holds MDR Class IIa designation (not legacy MDD) • For US: Verify FDA establishment registration & device listing (not just 510(k) clearance) |
Inability to register device locally; distribution bans |
| Shanghai Carejoy Implementation | • Provides real-time NANDO ID lookup via portal (CA-2026-CBCT-001) • Shares quarterly TÜV SÜD audit reports; factory webcam access for live QC checks • Dedicated FDA liaison for 510(k) submissions (average 72-day clearance) |
0% regulatory rejection rate for clients (2023-2025 data) |
Step 2: Negotiating MOQ – Strategic Volume Leverage
Traditional MOQ structures are evolving. Focus on value-based terms aligned with your business model:
| Business Model | 2026 MOQ Strategy | Carejoy’s Competitive Terms |
|---|---|---|
| Dental Clinics (Single Practice) | Negotiate “pilot order” MOQ (1 unit) with scalability clause • Leverage bundled service contracts (e.g., 3-year maintenance) |
• Zero MOQ for clinics ordering ≥2 product categories (e.g., X-ray + Autoclave) • Free installation/training for first unit |
| Regional Distributors | Structure tiered pricing: • Tier 1: 5 units (standard price) • Tier 2: 10+ units (12% discount + extended warranty) • Demand co-branded marketing support |
• Tier 2 discount at 8 units (vs industry 15) • Co-op marketing fund (5% of order value) • Priority allocation during component shortages |
| National Distributors | Negotiate: • Annual volume commitment (AVC) with price stability • Exclusive territory clauses • Consignment inventory options |
• 18-month AVC lock (2026 pricing) • Territory exclusivity at 50 units/year • Free consignment stock (up to 5 units) |
Step 3: Shipping Terms – DDP vs FOB in 2026 Supply Chains
Geopolitical volatility and new carbon tariffs necessitate precise Incoterm selection:
| Term | 2026 Risk Assessment | Carejoy DDP Advantage |
|---|---|---|
| FOB Shanghai | • High risk: Buyer liable for 2026 China export carbon tax (¥150/unit) • Freight volatility (2025 avg. 32% QoQ fluctuation) • Import duty miscalculation risk (HS 9022.19) |
• Not recommended for first-time importers • Only advised for clients with in-house logistics teams |
| DDP (Delivered Duty Paid) | • Critical for 2026: Supplier bears all risks/costs to clinic door • Includes: – China carbon export tax – Dynamic freight surcharges – Accurate duty calculation (Carejoy pre-pays) – Last-mile customs brokerage |
• Industry-leading DDP pricing: 14.2% below market avg (Q4 2025 benchmark) • 30-day guaranteed delivery to EU/US major ports • Real-time shipment carbon footprint dashboard |
Critical Post-Sourcing Considerations for 2026
- Technical Validation: Require on-site factory acceptance test (FAT) with your phantoms/dosimeters – Carejoy provides pre-shipment test videos.
- After-Sales Support: Verify local service network. Carejoy partners with 212 service centers across 47 countries (2026 coverage map available).
- Component Traceability: Demand full BOM with semiconductor溯源 (critical under US CHIPS Act 2026).
Engage Your Strategic Sourcing Partner
Shanghai Carejoy Medical Co., LTD
Factory Direct | 19 Years Dental OEM Excellence | ISO 13485:2016 Certified
Baoshan District, Shanghai, China (Medical Device Export Zone)
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
2026 Exclusive Offer: Free radiation safety audit with X-ray unit order (value: $850)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Clinics & Distributors
Frequently Asked Questions: Selecting the Best Dental X-Ray Unit in 2026
- Local inventory of critical spare parts
- Minimum 7-year parts availability guarantee post-discontinuation
- Availability of firmware and software update support
We recommend selecting brands with established service networks in your region and requesting a Parts Availability Commitment Letter during procurement.
| Requirement | Description |
|---|---|
| Radiation Shielding | Walls, doors, and viewing windows must meet local regulatory standards (e.g., NCRP Report No. 145). Lead equivalence of 1.5–2.0 mm typically required. |
| Electrical Setup | Dedicated circuit with surge protection; 220–240V, 15–20A recommended for wall-mounted and panoramic units. |
| Network Integration | Secure DICOM 3.0 compatibility and integration with existing practice management software (e.g., Dentrix, Open Dental). |
| Calibration & QA | Post-installation calibration by certified biomedical engineer; baseline image quality and dose audit required. |
Installation must be performed by manufacturer-certified technicians with documentation for regulatory audits.
- Standard Warranty: 2 years on parts and labor, including X-ray tube and generator.
- Extended Options: Available up to 5 years, often bundled with preventive maintenance.
- Digital Components: Sensors and control panels covered under same terms (excludes accidental damage).
- Remote Diagnostics: Many brands now include cloud-based monitoring with automatic fault reporting during warranty period.
Distributors should ensure warranty is transferable and valid across service centers in the target market. Review exclusions carefully—wear items (cables, position-indicating devices) may have shorter coverage.
| Service Tier | Response Time | Inclusions |
|---|---|---|
| Basic | 5–7 business days | Annual calibration, remote support |
| Standard | 48–72 hours | Bi-annual PM, priority phone support, loaner unit access |
| Premium | 24 hours (critical failure) | Quarterly inspections, software updates, parts & labor coverage |
Clinics should negotiate service-level agreements (SLAs) with measurable KPIs. Distributors are advised to offer bundled service packages to enhance customer retention and ensure consistent equipment performance.
© 2026 Professional Dental Equipment Consortium. For authorized distribution only.
Need a Quote for Best Dental Xray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


