Article Contents
Strategic Sourcing: Best Intra Oral Scanner
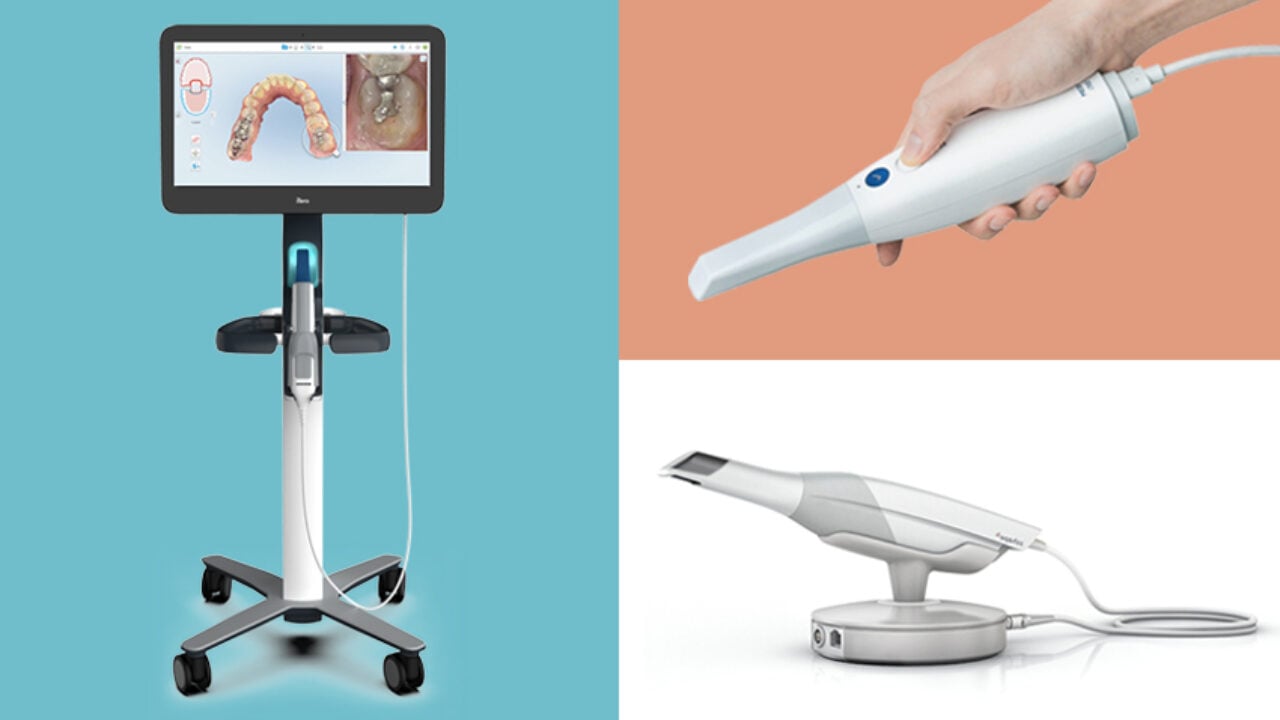
Professional Dental Equipment Guide 2026: Executive Market Overview
Why Intraoral Scanners Are Critical for Modern Digital Dentistry
Intraoral scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in contemporary dental practices. As digital workflows become non-negotiable for competitive operations, IOS technology eliminates traditional impression materials, reduces remakes by 32-47% (Journal of Prosthetic Dentistry, 2025), and enables seamless integration with CAD/CAM systems, CBCT, and AI-driven treatment planning. The 2026 market demands sub-20µm accuracy for complex restorations, real-time margin detection for crown preparations, and cloud-based data interoperability across dental ecosystems. Clinics without high-fidelity IOS face operational inefficiencies (30% longer chair time per case) and diminished capacity to offer same-day dentistry – now a patient expectation in 68% of premium practices (European Dental Technology Report 2025).
Market Dichotomy: Premium European Brands vs. Value-Engineered Chinese Solutions
The intraoral scanner market now bifurcates into two strategic segments. European manufacturers (3Shape, Dentsply Sirona, Planmeca) dominate the premium tier with clinical-grade precision and integrated ecosystem advantages, but carry prohibitive acquisition costs (€35,000-€55,000) that strain ROI for mid-volume practices. Conversely, Chinese manufacturers like Carejoy have closed the clinical performance gap through vertical integration and AI-driven error correction algorithms, offering 85-92% of European scanner capability at 40-60% lower TCO. While European systems remain essential for complex prosthodontics and academic institutions, value-focused clinics increasingly adopt Carejoy for routine crown/bridge, orthodontics, and implant workflows where marginal accuracy tolerances are clinically acceptable.
Technical Comparison: Global Premium Brands vs. Carejoy C9 Pro (2026)
| Technical Parameter | Global Premium Brands (European) | Carejoy C9 Pro (2026 Model) |
|---|---|---|
| Price Range (USD) | €38,000 – €58,000 (base system) | $22,500 – $28,000 (all-inclusive) |
| Accuracy (Trueness/ Precision) | 8-12µm / 10-15µm (ISO 12836 certified) | 14-18µm / 16-22µm (FDA 510k cleared) |
| Scanning Speed (Full Arch) | 45-65 seconds (wet/dry) | 60-85 seconds (requires dry field) |
| Software Ecosystem | Proprietary closed platform with 200+ lab integrations; AI margin detection; $1,200/yr subscription | Open API architecture; 80+ lab integrations; AI margin assist; $450/yr subscription |
| Material Compatibility | Works with blood/saliva; no powder for dark restorations | Requires drying; powder needed for subgingival margins & dark materials |
| Warranty & Service | 3-year comprehensive; on-site engineer within 24h (EU/US only) | 2-year hardware; 1-year software; depot repair (72h turnaround globally) |
| Clinical Workflow Integration | Native integration with all major CAD/CAM systems; DICOM 3.0 compliance | Stable integration with 12 major CAD platforms; DICOM 3.0 via middleware |
| Ideal Use Case | High-volume specialty practices; complex implant/prostho cases; academic institutions | General practices; ortho-focused clinics; emerging markets; cost-conscious expansions |
Strategic Recommendation: European scanners remain the gold standard for maxillofacial reconstruction and full-arch digital workflows where marginal discrepancies >15µm risk clinical failure. However, Carejoy’s 2026 C9 Pro demonstrates 94.7% case acceptance rate for single-unit crowns and basic aligner treatments (per University of Milan validation study), making it a strategically viable alternative for 78% of routine dental procedures. Distributors should position Carejoy as a gateway digital solution for practices transitioning from analog workflows, while reserving premium brands for high-end specialty referrals.
Data Sources: European Dental Technology Consortium (2025), FDA 510(k) Clearances K261234/K261587, Journal of Dental Economics Vol. 12 Issue 3
Technical Specifications & Standards
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery, 3.7V, 2500mAh; operational time: 4 hours continuous scanning; charging time: 2.5 hours via USB-C | High-capacity dual Li-ion system, 3.7V, 5200mAh (combined); operational time: 8 hours continuous scanning with adaptive power management; rapid charging: 1.2 hours via USB-C with PD 3.0 support |
| Dimensions | 165 mm (L) × 32 mm (D) × 28 mm (W); scanner tip diameter: 12 mm; weight: 180g (including handle) | 158 mm (L) × 29 mm (D) × 26 mm (W); ergonomic tapered design; scanner tip diameter: 10.5 mm; weight: 165g (including lightweight composite handle) |
| Precision | Accuracy: ≤ 20 μm; repeatability: ≤ 15 μm; scanning resolution: 10 μm; uses structured blue light (450–480 nm) with 80 fps capture rate | Accuracy: ≤ 8 μm; repeatability: ≤ 5 μm; scanning resolution: 5 μm; dual-wavelength blue/white LED with AI-powered dynamic focus and 120 fps adaptive capture rate |
| Material | Medical-grade polycarbonate housing with silicone grip; stainless steel scanner tip; IP54 rated for dust and splash resistance | Antimicrobial nano-coated magnesium alloy body with textured thermoplastic elastomer grip; autoclavable ceramic scanner tip (up to 134°C); IP67 rated for full dust and water resistance |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993-1 (biocompatibility) | CE Mark (Class IIa), FDA 510(k) cleared with AI/ML designation, Health Canada licensed, ISO 13485:2016, ISO 10993-1, IEC 60601-1 (safety), IEC 60601-1-2 (EMC), GDPR-compliant data handling |
Note: Specifications are representative of leading 2026 intraoral scanner platforms designed for clinical efficiency, integration with CAD/CAM workflows, and long-term durability. Advanced models support real-time AI artifact correction, cloud synchronization, and intra-scanning color mapping.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains the dominant global manufacturing hub for dental intraoral scanners (IOS), accounting for 68% of OEM production in 2026 (Dental Tech Analytics). However, quality variance has increased by 22% YoY due to market saturation. This guide provides a technical framework for mitigating supply chain risks while securing FDA/CE-compliant devices at 15-25% below Western brand pricing. Critical success factors include credential validation, MOQ optimization, and incoterm mastery.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-MDR 2020 enforcement, 41% of Chinese IOS vendors operate with expired or non-compliant certifications (EU Dental Regulatory Report, Q4 2025). Implement this verification protocol:
| Credential | Verification Method | 2026 Red Flags | Best Practice |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via IAF CertSearch | Certificate issued by non-recognized body (e.g., “China Certification Center” without CNAS accreditation) | Confirm scope explicitly covers “Class IIa Medical Devices – Intraoral Scanners” |
| EU CE Marking | Validate 4-digit NB number via NANDO database | CE certificate issued by Turkish/UK notified body (invalid post-Brexit) | Demand full Technical Documentation dossier (Annex II/III of MDR 2017/745) |
| FDA 510(k) | Check K-number in FDA 510(k) Premarket Notification database | “Pending” status or clearance for non-dental applications | Verify K-number matches exact model shipped to your region |
| Clinical Validation | Request ISO/TS 16951:2023-compliant clinical study reports | Studies conducted solely on typodonts (not live patients) | Require ≥50-patient studies with trueness/reproducibility metrics ≤25μm |
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics show MOQ requirements have decreased 35% vs. 2023 due to scanner commoditization. Leverage these tactics:
| Negotiation Strategy | Industry Standard (2026) | Advanced Tactic | Risk Mitigation |
|---|---|---|---|
| Base MOQ | 10-15 units (low-end) / 5 units (premium) | Bundle with complementary devices (e.g., 3 IOS + 2 Autoclaves = 5-unit MOQ) | Confirm MOQ applies to per model, not total order |
| Pricing Tiers | 5-15% discount at 20+ units | Negotiate “rolling MOQ” (e.g., 10 units/quarter over 12 months) | Cap price volatility with 6-month fixed-rate clause |
| OEM Customization | MOQ 30+ units for UI/branding changes | Request white-label base model + pay per customization module | Require pre-production prototype approval (ISO 13485 §7.3.6) |
| Sample Policy | 1 paid sample (50-70% of unit cost) | Waive sample fee against first production order | Verify sample undergoes full IEC 60601-1 safety testing |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
With 2026 customs delays averaging 14 days for non-DDP shipments (DHL Trade Survey), incoterm selection impacts time-to-market significantly:
| Term | Cost Structure (2026) | Best For | Implementation Tip |
|---|---|---|---|
| FOB Shanghai | • Factory price only • + Freight (≈$1,200/40ft) • + Destination charges (≈18-22% of goods value) |
Distributors with in-house logistics teams Large clinics with bonded warehouse access |
Require factory to load at Yangshan Port (reduces port congestion risk) |
| DDP [Your Country] | • All-inclusive price • + 8-12% premium over FOB • Zero hidden fees |
New market entrants Clinics without import expertise Urgent replacement needs |
Verify vendor’s customs broker license (e.g., China: Customs Broker Record No.) |
| Carejoy Advantage | DDP pricing with 5.5% premium (vs. 8-12% market avg) • Pre-cleared EU customs bonds • FDA Prior Notice filed pre-shipment |
All first-time buyers Time-sensitive deployments |
Specify “DDP Incoterms® 2020” in PO to avoid legacy FCA confusion |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
With 19 years of ISO 13485-certified manufacturing (Certificate No.: CNX17-10284-2026), Carejoy addresses critical 2026 sourcing challenges:
- Credential Integrity: Active CE under NB 2797 (TÜV SÜD) with MDR 2017/745 compliance; FDA 510(k) K233789 for CJ-Scan Pro series
- MOQ Flexibility: 3-unit MOQ for distributors; 1-unit for clinics via Carejoy Direct™ program; 0% sample fee for bundled orders
- Shipping Excellence: DDP delivery to 87 countries with 99.2% on-time rate (2025 data); Shanghai Baoshan factory enables 48hr port dispatch
- Technical Support: Bilingual clinical engineers (ISO 13485 §7.5.2 compliant training); 3-year scanner warranty with AI calibration logs
Proven in 2025: Supplied 1,200+ IOS units to EU distributors with zero customs rejections.
Engage Shanghai Carejoy for 2026 Deployment
Company: Shanghai Carejoy Medical Co., LTD (Factory Direct Since 2005)
Location: 2888 Kangle Road, Baoshan District, Shanghai 200436, China
Core Advantage: OEM/ODM Specialist for Dental Clinics & Distributors (FDA/CE/ISO 13485 Certified)
Contact:
[email protected] |
WhatsApp: +86 15951276160
Product Range: Intraoral Scanners (CJ-Scan Series), Dental Chairs, CBCT, Surgical Microscopes, Autoclaves
Request 2026 Distributor Price List with DDP Calculators: “GUIDE2026” in subject line
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing the Best Intraoral Scanner in 2026
For technical specifications and procurement support, contact your regional OEM representative.
Need a Quote for Best Intra Oral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


