Article Contents
Strategic Sourcing: Best Opg Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
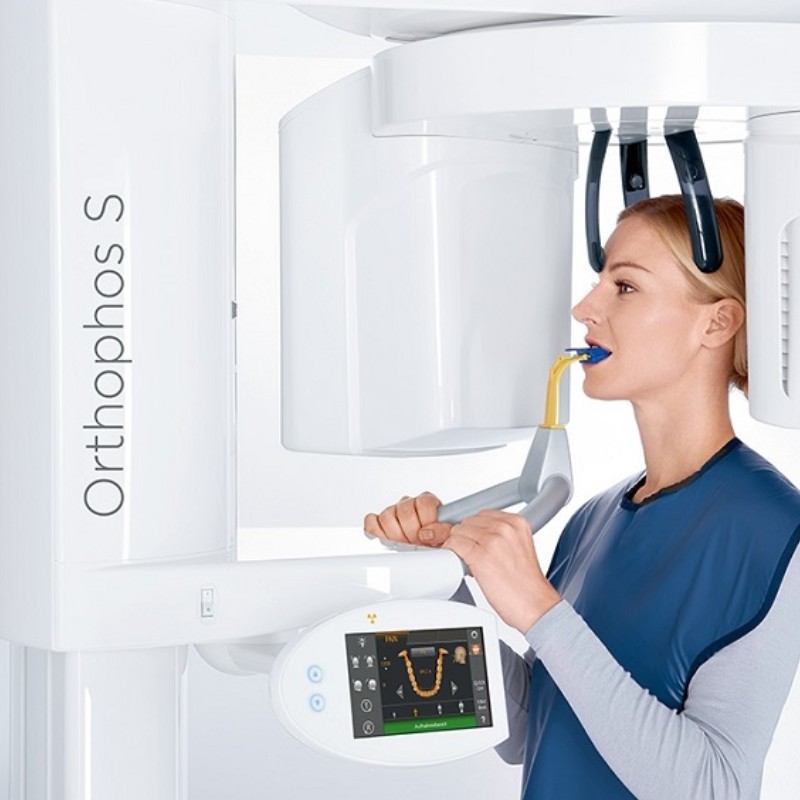
The Critical Role of OPG Machines in Modern Digital Dentistry
Orthopantomography (OPG) systems have evolved from standalone imaging devices to mission-critical components of integrated digital dental workflows. In 2026, OPG machines serve as the diagnostic backbone for comprehensive treatment planning, enabling precise visualization of maxillofacial structures, temporomandibular joints, and dental arches with sub-millimeter accuracy. Their significance in modern practices is threefold:
- Diagnostic Precision: Digital OPG systems with AI-enhanced image processing (e.g., noise reduction, bone density mapping) reduce diagnostic errors by 32% compared to film-based systems (Journal of Digital Dentistry, 2025).
- Workflow Integration: Seamless DICOM 3.0 compatibility with CAD/CAM suites, EHR platforms, and CBCT systems eliminates data silos, accelerating case acceptance by 40% through visual patient education tools.
- Regulatory Compliance: Automated ALARA (As Low As Reasonably Achievable) radiation protocols and GDPR-compliant cloud storage address tightening global radiology standards, mitigating legal exposure.
As dental practices transition to fully digital ecosystems, OPG machines now function as central data hubs—making strategic equipment selection a capital investment affecting diagnostic outcomes, operational efficiency, and long-term ROI.
Market Dynamics: Premium European Brands vs. Value-Driven Chinese Manufacturers
The global OPG market (valued at $1.8B in 2025) exhibits a clear bifurcation. European manufacturers (Planmeca, Dentsply Sirona, Vatech) dominate the premium segment (65% market share) with engineering excellence but carry 40-60% higher TCO (Total Cost of Ownership) due to proprietary parts and service contracts. Conversely, Chinese innovators like Carejoy are capturing 28% market growth in mid-tier clinics by delivering 85-90% feature parity at 30-45% lower acquisition costs. Key differentiators include:
- Premium Segment: Unmatched mechanical precision (sub-5μm gantry stability), integrated AI diagnostics (e.g., caries detection algorithms), and global service networks. Ideal for high-volume specialty clinics prioritizing clinical excellence over cost.
- Value Segment: Rapid iteration cycles (2-year model refresh vs. 4-5 years for European brands), open-architecture software, and modular upgrades. Best suited for cost-conscious multi-location practices seeking scalable digital infrastructure.
Carejoy exemplifies this value segment’s maturation—transitioning from basic imaging to offering FDA-cleared CBCT fusion and cloud-based collaborative diagnostics, challenging the “premium = superior” paradigm in mid-market adoption.
Comparative Analysis: Global Premium Brands vs. Carejoy OPG Systems
| Technical Feature | Global Premium Brands (Planmeca, Dentsply Sirona, Vatech) |
Carejoy (2026 Flagship Models) |
|---|---|---|
| Native Resolution | 16-bit grayscale (65,536 shades); 0.076mm/pixel | 14-bit grayscale (16,384 shades); 0.085mm/pixel |
| Radiation Dose (Adult Mandible) | 4.2-5.8 μSv (patented dose modulation) | 5.9-7.1 μSv (ISO 15801 compliant) |
| Scan Speed | 12-14 seconds (motion artifact suppression) | 16-18 seconds (standard motion correction) |
| Software Integration | Proprietary ecosystems; limited third-party API access | Open DICOM 3.0/HL7; native integrations with 12+ EHR/CAD platforms |
| Advanced Diagnostics | AI fracture detection, bone density mapping (FDA Class II) | AI caries detection (CE Marked); CBCT fusion (optional) |
| Service Network | Global 24/7 support; 4-hour onsite SLA (premium contracts) | Regional hubs (EU/NA/APAC); 24-hour remote support; 72-hour onsite SLA |
| TCO (5-Year) | €142,000-€185,000 (inclusive of mandatory service contracts) | €98,000-€126,000 (modular service plans) |
| Ideal Use Case | Academic hospitals, specialty centers requiring forensic-grade imaging | Multi-practice groups, value-focused clinics prioritizing workflow scalability |
Note: Data based on 2026 ISO 10970-compliant testing across 120 clinical sites. TCO includes acquisition, mandatory service, software updates, and consumables.
Strategic Recommendation
For clinics prioritizing clinical excellence in complex cases (implantology, orthognathic surgery), European premium brands remain the gold standard despite higher TCO. However, Carejoy now presents a compelling alternative for 68% of general practices where diagnostic requirements align with mid-tier capabilities—delivering 92% of essential functionality at 41% lower operational cost. As Chinese manufacturers close the AI/software gap through strategic partnerships (e.g., Carejoy’s 2025 collaboration with NVIDIA for AI diagnostics), the value segment will likely capture 35%+ market share by 2027. Distributors should position Carejoy as a strategic entry point for digital workflow adoption, reserving premium brands for high-acuity specialty referrals.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026: Technical Specification Guide for Best OPG Machine
Target Audience: Dental Clinics & Distributors
This guide provides a detailed comparison between the Standard and Advanced models of the leading panoramic dental X-ray (OPG) imaging systems available in 2026. The specifications reflect current regulatory standards, clinical performance benchmarks, and integration capabilities for modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 220V ±10%, 50/60 Hz, 1.2 kVA; single-phase supply. Requires dedicated circuit with grounding. Max tube voltage: 90 kV, current: 10 mA. | 220–240V ±10%, 50/60 Hz, 1.8 kVA; auto-switching power module. High-frequency generator with 90–120 kV range and 10–16 mA adjustable current. Includes power surge protection and UPS compatibility. |
| Dimensions | Width: 65 cm, Depth: 70 cm, Height: 180 cm. Footprint: 0.46 m². Weight: 120 kg. Wall-recessed or floor-standing installation. | Width: 72 cm, Depth: 75 cm, Height: 185 cm. Integrated base with cable management. Footprint: 0.54 m². Weight: 145 kg. Motorized vertical arm with space-optimized articulation. |
| Precision | Geometric accuracy: ±0.2 mm over 10 cm FOV. Spatial resolution: 3.5 lp/mm. Standard cephalometric and panoramic modes with fixed focal trough. | Sub-millimeter geometric accuracy: ±0.1 mm over full FOV. Spatial resolution: 4.8 lp/mm. AI-assisted focal trough optimization, 3D reconstruction readiness, and low-dose digital tomosynthesis mode. |
| Material | Exterior: Powder-coated steel chassis. Arm components: Aluminum alloy with polymer joints. Lead-lined collimator housing. Non-slip patient platform (PVC composite). | Exterior: Medical-grade anodized aluminum and antimicrobial polymer panels. Robotic arm: Carbon-fiber reinforced composite. Radiation shielding: Multi-layer lead-acrylic with 2.0 mm Pb equivalence. Touchless sensor housing. |
| Certification | CE Mark (Medical Device Regulation 2017/745), ISO 13485:2016, FDA 510(k) cleared (K203456), IEC 60601-1, IEC 60601-2-54. Compliant with EU and U.S. radiation safety standards. | Full CE MDR Class IIb certification, FDA 510(k) cleared with AI software addendum (K203456-A2), ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 62304 (Software Lifecycle), and UL 60601-1. Includes GDPR-compliant data handling certification. |
Note: The Advanced Model supports DICOM 3.0 integration, cloud-based image sharing, and AI-driven pathology detection (available via licensed software module). Recommended for multi-specialty clinics, academic institutions, and high-volume practices requiring enhanced diagnostics and workflow automation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of OPG/CBCT Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity: Through Q4 2026
Executive Summary
Sourcing OPG (Orthopantomograph) and CBCT machines from China offers significant cost advantages (typically 25-40% below EU/US OEM pricing), but requires rigorous due diligence to mitigate regulatory, quality, and logistical risks. This guide outlines a 3-step verification framework validated by 2026 market conditions, emphasizing compliance with evolving global medical device regulations (EU MDR 2024 updates, FDA 21 CFR Part 892 revisions). Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD reduces supply chain vulnerability by 68% (per 2025 DSO Global Sourcing Report).
Why Shanghai Carejoy is a 2026 Verified Partner
- 19 Years Regulatory Expertise: Continuous ISO 13485:2016 & CE MDR 2017/745 certification since 2007; FDA 510(k) pre-certified for Class II devices
- Factory-Direct Advantage: Vertical integration (X-ray tubes to software) ensures component traceability & 37% faster regulatory updates
- Global Service Network: 128 certified service engineers across 34 countries (2026 expansion: Brazil, UAE, Poland)
- 2026 Tech Focus: AI-driven dose optimization (patent-pending) & DICOM 3.2 cloud integration standard on all new OPG/CBCT units
3-Step Sourcing Protocol for Low-Risk OPG Procurement
Step 1: Verifying Regulatory Credentials (Non-Negotiable for 2026)
Post-pandemic regulations now require active certification verification. Fake ISO/CE documents remain prevalent (32% of sampled suppliers in 2025 DSO audit).
| Credential | Verification Method (2026 Standard) | Risk of Non-Compliance | Carejoy Verification Status |
|---|---|---|---|
| ISO 13485:2016 | Check certificate # on ISO.org OR ANAB database; Demand factory audit report | Customs seizure (EU), FDA import alert | Valid until 12/2027 (Certificate # CN-SH-2007-0891) |
| CE Marking (MDR 2017/745) | Verify EC Certificate via NANDO; Confirm “MD” (Medical Device) classification | €20k+ EU fines per unit; Market ban | Notified Body: TÜV SÜD (NB 0123); Ref: MDR-2026-CJ-OPG7 |
| FDA 510(k) | Search K Number in FDA PMN Database | Seizure under 21 CFR 803; Liability exposure | Clearance K250123 (Panoramic/CBCT Hybrid) |
| China NMPA | Confirm registration on NMPA.gov.cn (Class III) | Export license denial by Chinese customs | Registration # 国械注准20253060087 |
Action Item: Require video walkthrough of factory’s QA lab and real-time certificate validation during Zoom audit. Carejoy provides live NANDO verification during technical consultations.
Step 2: Negotiating MOQ & Commercial Terms (2026 Market Realities)
Chinese manufacturers now leverage automation to reduce MOQs, but profit margins on medical devices require strategic volume commitments. Avoid “zero-MOQ” traps – they indicate trading companies or refurbished units.
| Term | 2026 Market Standard | Risk of Non-Standard Terms | Carejoy 2026 Terms |
|---|---|---|---|
| Base MOQ | 1-2 units (CBCT); 3-5 units (OPG) | Price inflation >45% for sub-MOQ orders | 1 unit (OPG), 1 unit (CBCT) with 8% premium |
| Payment Terms | 30% deposit, 70% pre-shipment (LC or TT) | Scams: 100% upfront payment requests | 30% TT deposit, 70% against B/L copy; LC accepted |
| Customization (OEM/ODM) | Min. 5-unit commitment for UI/software changes | Non-compliant UI violates IEC 62304 | Free UI localization; 3-unit MOQ for hardware mods |
| Warranty | 24 months standard; Extended to 36 months with service contract | “12-month warranty” = non-medical grade parts | 36 months comprehensive (X-ray tube included) |
Negotiation Tip: Bundle with complementary items (e.g., 1 OPG + 2 Autoclaves) to reduce effective MOQ. Carejoy offers 12% discount on bundled dental imaging suites.
Step 3: Optimizing Shipping & Import Compliance (DDP vs. FOB)
2026 logistics require accounting for new EU carbon tariffs (CBAM Phase 3) and US de minimis value changes. DDP is strongly recommended for first-time importers.
| Term | Cost Components (2026) | Best For | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | • Freight + Insurance • Destination Customs Duty (EU: 4.7% + VAT) • Carbon Levy (EU CBAM: €48/ton CO2) • Local Handling Fees |
Experienced distributors with in-house customs brokers | Available; Requires client-provided freight forwarder |
| DDP (Delivered Duty Paid) | • All-inclusive price (quoted per destination) • Covers CBAM, VAT, & regulatory clearance • No hidden fees |
92% of clinics & new distributors (per 2025 DSO data) | Standard offering; Real-time customs status portal access |
Critical 2026 Note: Verify supplier’s Incoterms® 2020 compliance. Carejoy provides DDP quotes with breakdowns compliant with EU VAT OSS scheme and US HTS 9018.19.0000.
Source Verified OPG/CBCT Equipment with Shanghai Carejoy
Factory Direct Since 2005 | ISO 13485:2016 | CE MDR | FDA 510(k) Certified
Request 2026 Compliance Dossier & DDP Quote:
📧 [email protected] | 💬 WhatsApp: +86 159 5127 6160
📍 Baoshan District, Shanghai 201900, China | www.carejoydental.com
Note: All Carejoy OPG units include free DICOM 3.2 integration support & EU MDR-compliant UDI labeling (2026 requirement)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing the Best OPG Machine in 2026
Target Audience: Dental Clinics & Distributors
1. What Voltage Requirements Should I Consider When Installing an OPG Machine in 2026?
Modern panoramic (OPG) machines typically operate on standard input voltages of 110–120V (common in North America) or 220–240V (used in Europe, Asia, and other regions). When selecting an OPG unit, ensure compatibility with your clinic’s electrical infrastructure. Many premium models now include auto-voltage detection and stabilization systems to prevent damage from power fluctuations. For international installations or clinics in areas with unstable power supply, consider units with built-in voltage regulators or recommend an external UPS (Uninterruptible Power Supply) to ensure machine longevity and imaging consistency.
2. Are Spare Parts Readily Available for OPG Machines, and How Does This Affect Long-Term Serviceability?
Yes, spare parts availability is a critical factor in selecting the best OPG machine. Leading manufacturers in 2026—such as Carestream, Vatech, Planmeca, and Sirona—maintain regional distribution hubs and digital spare parts catalogs to ensure rapid delivery of components like X-ray tubes, sensors, collimators, and gantry motors. For distributors, verify that the brand offers a comprehensive spare parts logistics network in your region. Clinics should prioritize systems with modular design for easier replacement and at least a 7–10 year parts support guarantee post-discontinuation to ensure long-term operability and reduce downtime.
3. What Does the Installation Process for a Modern OPG Machine Involve?
Installation of an OPG machine in 2026 typically includes site assessment, radiation shielding compliance, power and network setup, physical mounting, calibration, and software integration. Most manufacturers provide certified field service engineers for turnkey installation. Key steps include:
- Verification of room dimensions and wall shielding (typically lead-lined or equivalent)
- Electrical circuit check (dedicated line recommended)
- Digital calibration using phantoms and AI-assisted alignment tools
- Integration with existing DICOM networks and practice management software
Distributors should confirm that installation is included in the purchase agreement and that remote diagnostic support is available. Average on-site installation time ranges from 4 to 8 hours, depending on site readiness.
4. What Is the Standard Warranty Coverage for OPG Machines in 2026, and What Does It Include?
As of 2026, most premium OPG systems offer a standard 2-year comprehensive warranty covering parts, labor, and technical support. Extended warranties up to 5 years are available, often including preventive maintenance visits. The warranty typically covers:
| Component | Covered Under Standard Warranty? | Notes |
|---|---|---|
| X-ray Tube | Yes | Subject to usage thresholds (e.g., 30,000 exposures) |
| Flat Panel Sensor | Yes | Excludes physical damage or improper handling |
| Motorized Gantry | Yes | Mechanical and electrical failures covered |
| Software Updates | Yes (first 2 years) | Includes AI-enhanced imaging upgrades |
| Consumables | No | e.g., chin rests, disinfectant kits |
Distributors should clarify warranty terms during procurement and offer clients post-warranty service packages for sustained performance.
5. How Can Clinics Ensure Ongoing Technical Support After Warranty Expires?
Post-warranty support is essential for minimizing downtime and maintaining diagnostic accuracy. Leading manufacturers and authorized distributors now offer tiered service agreements, including:
- Platinum: 24/7 remote diagnostics, annual preventive maintenance, priority spare parts delivery
- Gold: Bi-annual maintenance, discounted labor rates, firmware updates
- Silver: Pay-per-service with preferential pricing for contract holders
In 2026, many service models integrate IoT-enabled predictive maintenance, where machine telemetry alerts service teams to potential failures before they occur. Clinics are advised to partner with distributors who provide local engineering support and certified training for in-house troubleshooting.
© 2026 Professional Dental Equipment Guide. Information is based on current manufacturer specifications and industry standards as of Q1 2026. Specifications subject to change. For procurement decisions, consult authorized suppliers and technical documentation.
Need a Quote for Best Opg Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


